B4PymPm
CAS Number 1030380-51-8
Charge Transport Layer Materials, Electron Transport Layer Materials, High Purity Sublimed Materials, Materials,B4PymPm, ETL and HBL material in OLEDs and photovoltaics
Significantly enhances charge-carrier mobility and transport
Overview | Specifications | MSDS | Literature and Reviews
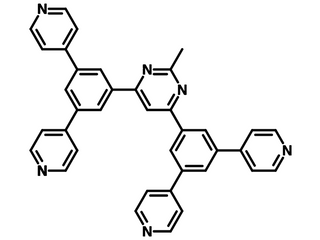
B4PymPm is an isomer to B2PymPm and B3PymPm. It has a 2-methylpyrimidine core structure with four pyridine pendants. It is electron-deficient and can be used in OLEDs and photovoltaics (e.g. perovskite solar cells) as an electron-transporting or hole-blocking layer material.
Due to its intermolecular hydrogen bonding, B4PymPm molecules self-assemble in a horizontal orientation - parallel to the substrate, with a significantly large anisotropy. This self-assembly gives high molecular stacking in films with high π-orbital overlaps, which can significantly enhance charge-carrier mobility and transport.
General Information
| CAS number | 1030380-51-8 |
|---|---|
| Full name | 4,6-Bis(3,5-di(pyridin-4-yl)phenyl)-2-methylpyrimidine, 4,6-Bis(3,5-di-4-pyridinylphenyl)-2-methylpyrimidine |
| Chemical formula | C37H26N6 |
| Molecular weight | 554.64 g/mol |
| Absorption | λmax 250 nm in DCM |
| Fluorescence | λmax 410 nm in Film |
| Synonyms | 4,6-Bis(3,5-di-4-pyridinylphenyl)-2-methylpyrimidine |
| HOMO/LUMO |
HOMO = −7.3 eV LUMO = −3.7 eV [6] |
| Classification / Family | Pyrimidine derivatives, Highly efficient light-emitting diodes, Organic electronics, Electron-transport layer (ETL) materials, Hole-blocking layer (HBL) materials, Sublimed materials. |
Product Details
| Purity | Sublimed >99.0% (HPLC) |
|---|---|
| Melting point | 374 °C (lit.) |
| Appearance | White crystals/powder |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials.
Chemical Structure
Device Structure(s)
| Device structure | ITO (110 nm)/HAT-CN (10 nm)/TAPC (40 nm)/TCTA (10 nm)/mCP (10 nm)/mCP:B4PyMPM:15 wt% FIrpic (20 nm)/B4PyMPM (50 nm)/Liq (0.8 nm)/Al (120 nm) [2] |
|---|---|
| Color | Blue |
| Max. Power Efficiency | 79.8 lm W−1 |
| Max. Current Efficiency | 41.3 cd/A |
| Max. EQE | 17.3% |
| Device structure | ITO (110 nm)/TAPC (40 nm)/TCTA (10 nm)/mCP (10 nm)/mCP:50 wt% B4PyMPM:15 wt% FIrpic:0.2 wt% PO-01* (20 nm)/B4PyMPM (50 nm)/Liq (0.8 nm)/Al (120 nm) [2] |
|---|---|
| Color | White |
| Max. Power Efficiency | 105.0 lm W−1 |
| Max. Current Efficiency | 83.6 cd/A |
| Max. EQE | 28.1% |
| Device structure | ITO (70 nm)/TAPC (75 nm)/TCTA (10 nm)/ TCTA:B4PYMPM:8 wt% Ir(ppy)2tmd (30 nm)/B4PYMPM (50 nm)/LiF (0.7 nm)/Al (100 nm) [3] |
|---|---|
| Color | Yellow |
| Max. Power Efficiency | 152.5 lm W−1 |
| Max. EQE | 30.4% |
| Device structure | ITO/HAT-CN (5 nm)/TAPC (30 nm)/TCTA (8 nm)/26DCzPPy:PO-01 (4 wt%, 2 nm)/26DCzPPy:B4PyMPM:FIrpic (1:1, 15 wt%, 20 nm)/ B4PyMPM (15 nm)/Bphen:LiH 0.1 wt% (25 nm)/Al (120 nm) [4] |
|---|---|
| Color | White |
| Max. Power Efficiency | 95.5 lm W−1 |
| Max. Current Efficiency | 82.0 cd/A |
| Max. EQE | 28.5% |
*For chemical structure information, please refer to the cited references
MSDS Documentation
Literature and Reviews
- Influence of Substituted Pyridine Rings on Physical Properties and Electron Mobilities of 2-Methylpyrimidine Skeleton-Based Electron Transporters, H, Sasabe et al., Adv. Funct. Mater., 21, 336–342 (2011); DOI: 10.1002/adfm.201001252.
- White Organic LED with a Luminous Efficacy Exceeding 100 lm W−1 without Light Out-Coupling Enhancement Techniques, S. Wu et al., Adv. Funct. Mater., 27, 1701314 (2017); DOI: 10.1002/adfm.201701314.
- Highly Efficient, Conventional, Fluorescent Organic Light-Emitting Diodes with Extended Lifetime, H. Kim et al., Adv. Mater., 29, 1702159 (2017); DOI: 10.1002/adma.201702159.
