Titanium(IV) Sulfide (TiS2) Powder and Crystal
CAS Number 12039-13-3
2D Materials, Low Dimensional Materials, Transition Metal Chalcogenides (TMCs), Transition Metal Dichalcogenides
Please choose the appropriate MSDS from the list below. Links will open in a new window. If your browser does not support PDFs, you will be prompted to download the file instead.
Low price, high purity 2D metal titanium(IV) sulfide powder and crystals
For the development of next-generation electronics, optoelectronics, and nanotechnology
Titanium(IV) sulfide (also known as titanium disulfide TiS2), CAS number 12039-13-3, is highly stable. It is the most lightweight and low-cost Group IV TMDC material. TiS2 is known to be a semimetal in when in bulk form with an indirect band-overlap of about 0.12 eV, and a semiconductor when strain or pressure is applied in the monolayer form. With a higher degree of overlap of the valence and conduction bands, its semi-metallic nature intensifies when compression is applied. However, the energetic overlap is lifted and a semimetal-to-semiconductor transition occurs under pressure or strains.
High Purity
≥99.999% Crystal Purity
Worldwide shipping
Quick and reliable shipping
Low Cost
Low Cost Titanium(IV) Sulfide
Powder & Crystal
Different Forms of Titanium(IV) Sulfide
TiS2 crystallises in an octahedral (1T) phase, which is more energetically stable compared to its hexagonal (2H) phase. Titanium(IV) sulfide has been successfully employed as an electrode material for rechargeable sodium and lithium ion batteries.
We supply low price titanium(iv) sulfide in several different forms for a range of applications.

Titanium(IV) Sulfide Powder
Can be used for preparation of titanium(IV) sulfide nanoplates and ultrathin films
Sold by weight
≥99.995% purity
From £350

Titanium(IV) Sulfide Crystals by Size
Can be used to produce single or few-layer titanium(IV) sulfide sheets via mechanical or liquid exfoliation
Small (≥10 mm2) or medium (≥25 mm2) crystals available*
≥ 99.999% purity
From £520
*Typical representative size, areas/dimensions may vary
Bulk single titanium(IV) sulfide crystal is most commonly used as sources from which single or few-layer sheets can be obtained via either mechanical or liquid exfoliation.
Platinum FET Test Chips for 2D Materials

- Affordable
- World-Wide Shipping
- Dual Channel Electrodes
Buy Online £200
Titanium(IV) sulfide powder can also be used to prepare TiS2 nanosheets and nanoparticles by liquid-exfoliation (normally assisted by sonication).
Key Product Data
- High purity, low price titanium(IV) sulfide
- Available as a powder or as individual crystal
- Can be used to produce single or few-layer sheets
- Free worldwide shipping on qualifying orders
Structure of Titanium(IV) Sulfide
A single sheet of TiS2 is formed by a titanium atom layer sandwiched between two layers of sulfur atoms that are covalently bonded to the titanium atoms. Like other Group IV transition metal dichalcogenides, TiS2 crystallises in an octahedral (1T) phase, which is more energetically stable compared to its hexagonal (2H) phase.
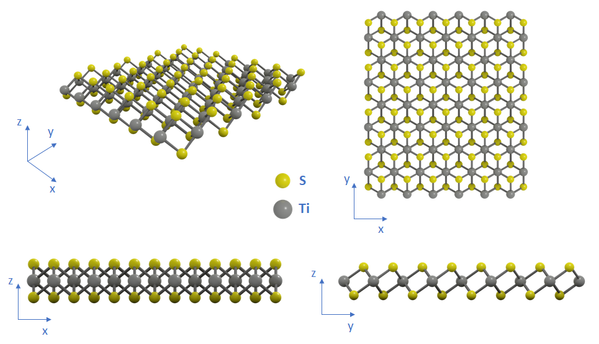
Properties of 2D Titanium(IV) Sulfide
After exfoliation of crystals or powder, titanium(IV) sulfide typically has the following properties:
- Hexagonal (1T) structure (space group: P3m1)
- Light weight, highly stable and low-cost
- Known to be a semimetal in when in bulk form and a semiconductor when strain or pressure is applied in the monolayer form
Applications of Titanium(IV) Sulfide
Titanium(IV) sulfide single crystals can be used to prepare monolayer and few-layer TiS2 by mechanical or liquid exfoliation. Titanium(IV) sulfide powder is suitable for liquid chemical exfoliation to prepare TiS2 nanosheets and nanoparticles down to few-layer films.
Monolayer titanium(IV) sulfide is the lightest member of the transition metal dichalcogenide family. It has promising applications in energy conversion and storage systems. 2D titanium(IV) sulfide has also been used in supercapacitors, and ultrafast fiber laser applications.
Two-dimensional thin layer nanosheets made from chemically-exfoliated titanium(IV) sulfide powder are generally used as electrode materials in storage batteries. Atomic-layered TiS2 quantum dot materials prepared from liquid exfoliation of TiS2 powder can also be used as electrocatalysts for hydrogen evolution reactions (HER).
TiS2 enables desalination of the sea water by the removal of sodium cations involving intercalation mechanism without employing an ion exchange membrane.
Literature and Reviews
- Electronic Properties and Chemical Reactivity of TiS2 Nanoflakes, C. Cucinotta et al., J. Phys. Chem. C, 119, 15707−15715 (2015); DOI: 10.1021/acs.jpcc.5b03212
- Tracking the Chemical and Structural Evolution of the TiS2 Electrode in the Lithium-Ion Cell Using Operando X‑ray Absorption Spectroscopy, L. Zhang et al., Nano Lett., 18, 4506−4515 (2018); DOI: 10.1021/acs.nanolett.8b01680.
- Room Temperature and Aqueous Solution-Processed 2D TiS2 as Electron Transport Layer for Highly Efficient and Stable Planar n-i-p Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 10 (17), 14796-14802 (2018); DOI: 10.1021/acsami.8b03225.
- Intercalation and lattice expansion in titanium disulfide, M. Whittingham et al., J. Chem. Phys. 62, 1588 (1975); doi: 10.1063/1.430581.
- Thio sol–gel synthesis of titanium disulfide thin films and nanoparticles using titanium(IV) alkoxide precursors, A. Let et al., J. Phys. Chem. Solids 68, 1428–1435 (2007); doi:10.1016/j.jpcs.2007.03.001.
- Unveiling two-dimensional TiS2 as an insertion host for the construction of high energy Li-ion capacitors, J. Mater. Chem. A, 5, 9177 (2017); DOI: 10.1039/c7ta01594a.
- TiS2 nanoplates:Ahigh-rateandstable electrode material for sodium ion batteries, Y. Liu et al., Nano Energy, 20, 168–175 (2016); doi: 10.1016/j.nanoen.2015.12.028.
- Titanium Disulfide: A Promising Low-Dimensional Electrode Material for Sodium Ion Intercalation for Seawater Desalination, P. Srimuk et al., Chem. Mater., 29, 9964−9973 (2017); DOI: 10.1021/acs.chemmater.7b03363.
- TiS2 Monolayer as an Emerging Ultrathin Bifunctional Catalyst: Influence of Defects and Functionalization, T. Das et al., ChemPhysChem, 20, 608–61 (2019); DOI: 10.1002/cphc.2018010.
- Strain-induced semimetal-to-semiconductor transition and indirect-to-direct band gap, transition in monolayer 1T-TiS2, C. Xu et al., RSC Adv., 2015, 5, 83876 (2015); DOI: 10.1039/c5ra16877e.
- Strain-induced enhancement of thermoelectric performance of TiS2 monolayer based on first-principles phonon and electron band structures, G. Li et al., Nanotechnology 29, 015204 (2018); doi: 10.1088/1361-6528/aa99ba.
- High Performance Pseudocapacitor Based on 2D Layered Metal Chalcogenide Nanocrystals, G. Muller et al., Nano Lett., 15, 1911−1917 (2015); DOI: 10.1021/nl504764m.
- 2D TiS2 Layers: A Superior Nonlinear Optical Limiting Material, S. Varma et al., Adv. Optical Mater., 5, 1700713 (2017); DOI: 10.1002/adom.201700713.
Technical Data
| CAS Number | 12039-13-3 |
| Chemical Formula | TiS2 |
| Molecular Weight | 111.987 g/mol |
| Bandgap | N/A |
| Preparation | Synthetic - Chemical Vapour Transport (CVT) |
| Structure | Octahedral (1T) |
| Electronic Properties | Semimetal, Diamagnetic |
| Melting Point | N/A |
| Colour | Golden yellow |
| Synonyms | Titanium disulfide, Bis(sulfanylidene)titanium |
| Classification / Family | Transition metal dichalcogenides (TMDCs), Nano-electronics, Nano-photonics, Photovoltaic, Materials science |
Product Details
| Form | Purity |
| Powder | ≥99.995% |
| Crystal | ≥99.999% |
MSDS Documents
Pricing Table
| Product Code | Form | Size/Weight* | Price |
| M2147C1 | Powder | 1 g | £350 |
| M2147A10 | Crystal | Small (≥10 mm2) | £520 ea. |
| M2147A25 | Crystal | Medium (≥25 mm2) | £850 ea. |
*typical representative size, areas/dimensions may vary

 Titanium(IV) sulfide powder
Titanium(IV) sulfide powder

















































































