Tungsten Disulfide (WS2) Crystal
CAS Number 12138-09-9
2D Materials, Low Dimensional Materials, Materials, Transition Metal Chalcogenides (TMCs),Tungsten Disulfide Crystal, high purity 2D semiconducting TMDCs material
For applications in light-emitting devices, solar cells, and photodetectors
Technical Data | MSDS | Structure | Literature and Reviews | Related Products | Resources and Support
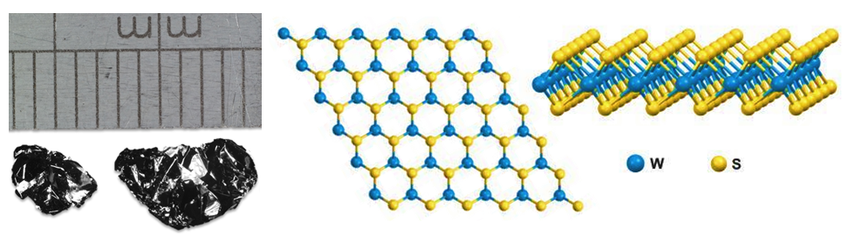
Like other TMDC family members, tungsten disulfide (WS2, CAS number 12138-09-9) has a two-dimensional structure. It consists of a layer of hexagonally-arranged tungsten atoms positioned between two layers of hexagonally-arranged sulfur atoms.
Individual layers are held together by Van der Waals forces. This makes it possible for single to few-layer ultra-thin films to be prepared from bulk crystals using mechanical exfoliation techniques. The primitive unit cell of WS2 consists of one tungsten atom and two sulfur atoms, arranged in a trigonal prismatic configuration.
High Purity
≥99.999% Crystal Purity
2D Semiconductor
2D semiconducting TMDCs material
Low Cost
Low Cost Tungsten Disulfide
Applications
Used to prepare monolayer and few-layer
With a photoluminescence (PL) quantum yield that is almost two orders of magnitude higher at room temperature than MoS2, WS2 is one of the most popular 2D semiconducting TMDCs materials, and is widely explored for applications in light-emitting devices, solar cells, and photodetectors.
Technical Data
| CAS Number | 12138-09-9 |
| Chemical Formula | WS2 |
| Molecular Weight | 247.97 g/mol |
| Bandgap | 1.4 - 2.01 eV [1] |
| Preparation | Synthetic - Chemical Vapor Transport (CVT) |
| Structure | Hexagonal |
| Electronic Properties | 2D semiconductor |
| Melting point | 1250 °C (lit.) |
| Color | Black |
| Synonyms | Tungsten sulfide, Tungsten sulfide, Tungsten(IV) sulfide |
| Classification / Family | Transition metal dichalcogenides (TMDCs), 2D semiconductor materials, Nano-electronics, Nano-photonics, Materials science |
Product Details
| Form | Purity |
|---|---|
| Crystal | ≥99.999% |
Pricing Table
| Product Code | Form | Size* | Quantity (EA) | Price |
|---|---|---|---|---|
| M2110A10 | Crystal | Small (≥10 mm2) | 1 | £520 |
| M2110A25 | Crystal | Medium (≥25 mm2) | 1 | £850 |
| M2110A00 | Crystal | Large** (≥100 mm2) | 1 | £1750 |
*Typical representative size, areas/dimensions may vary
**Item with a lead time of 2 - 3 weeks, please contact for more information
MSDS Document
Structure of Tungsten Disulfide Crystal
Literature and Reviews
- Controlled Synthesis and Transfer of Large-Area WS2 Sheets: From Single Layer to Few Layers, A. Elı´as et al., ACS Nano, 7 (6), 5235–5242 (2013); DOI: 10.1021/nn400971k.
- Extraordinary Room-Temperature Photoluminescence in Triangular WS2 Monolayers, H. R. Gutiérrez et al., Nano Lett., 13 (8), 3447–3454 (2013); DOI: 10.1021/nl3026357.
- Controlled Growth of High-Quality Monolayer WS2 Layers on Sapphire and Imaging Its Grain Boundary, Y. Zhang et al., ACS Nano, 7 (10), 8963–8971 (2013); DOI: 10.1021/nn403454e.
Related Products
We stock a wide range of 2D materials available to purchase online. Please contact us if you cannot find what you are looking for.



