Tin(IV) Selenide (SnSe2) Powder and Crystal
CAS Number 20770-09-6
2D Materials, Low Dimensional Materials, Materials, Post-Transition Metal Chalcogenides (PTMCs),Low price, high purity 2D metal tin(IV) selenide powder and crystals
For the development of next-generation electronics, optoelectronics, and nanotechnology
Technical Data | MSDS | Structure | Literature and Reviews | Related Products | Resources and Support
Tin(IV) selenide (also known as tin diselenide SnSe2), CAS number 20770-09-6, is a family member of two-dimensional layered transition metal dichalcogenides (TMDCs) semiconductors. Within each layer, every six selenium atoms are located at the corners of an octahedron, and feature an inversion symmetry (with respect to the central tin atom). The layered structure (bound by the weak Van der Waals forces) allows exfoliation in both solid and liquid forms to peel off layers from bulk crystals or powder.
High Purity
≥99.999% Crystal Purity
Worldwide shipping
Quick and reliable shipping
Low Cost
Low Cost Tin(IV) Selenide
Powder & Crystal
Different Forms of Tin(IV) Selenide
Outperforming most other 2D layered materials (such as MoS2 and WSe2), atomic layered SnSe2 exhibits high photoresponsivity and a very fast rise and fall response speed. This shows that few-layer SnSe2 is a promising active 2D material for electronic and optoelectronic applications.
SnSe2 is an earth-abundant semiconductor with an n-type binary nature. The band gap of SnSe2 can be tuned from bulk to few-layer thin films with a wide electromagnetic spectrum range (from 1 - 2 eV). This makes it an attractive 2D material for various photoelectronic applications.
We supply low price tin(iv) selenide in several different forms for a range of applications.

Tin(IV) Selenide Powder
Can be used for preparation of tin(IV) selenide nanoplates and ultrathin films
Sold by weight
≥99.995% purity
From £220
Tin(IV) Selenide Crystals by Size
Can be used to produce single or few-layer tin(IV) selenide sheets via mechanical or liquid exfoliation
Small (≥10 mm2) or medium (≥25 mm2) crystals available*
≥99.999% purity
From £520
*Typical representative size, areas/dimensions may vary
Bulk single tin(IV) selenide crystal is most commonly used as sources from which single or few-layer sheets can be obtained via either mechanical or liquid exfoliation.
Tin(IV) selenide powder can also be used to prepare SnSe2 nanosheets and nanoparticles by liquid-exfoliation (normally assisted by sonication).
Technical Data
| CAS Number | 20770-09-6-1 |
| Chemical Formula | SnSe2 |
| Molecular Weight | 276.63 g/mol |
| Bandgap | 1.07 - 1.69 eV |
| Preparation | Synthetic - Chemical Vapor Transport (CVT) |
| Structure | Hexagonal (2H) |
| Electronic Properties | 2D semiconductor |
| Melting Point | 650 °C |
| Color | Metallic black |
| Synonyms | Tin diselenide, Stannic selenide |
| Classification / Family | Transition metal dichalcogenides (TMDCs), 2D semiconductor materials, NIR band-gap, Nano-electronics, Nano-photonics, Transistors, Photovoltaics, Materials science |
Product Details
| Form | Purity |
|---|---|
| Powder | ≥99.995% |
| Crystal | ≥99.999% |
Pricing Table
| Product Code | Form | Size/Weight* | Price |
|---|---|---|---|
| M2115C1 | Powder | 500 mg | £220 |
| M2115C1 | Powder | 1 g | £350 |
| M2115A10 | Crystal | Small (≥10 mm2) | £520 ea. |
| M2115A25 | Crystal | Medium (≥25 mm2) | £850 ea. |
| M2115A00 | Crystal | Large (≥100 mm2) | £1800 ea. |
*Typical representative size, areas/dimensions may vary
Shipping is free for qualifying orders.
MSDS Documents
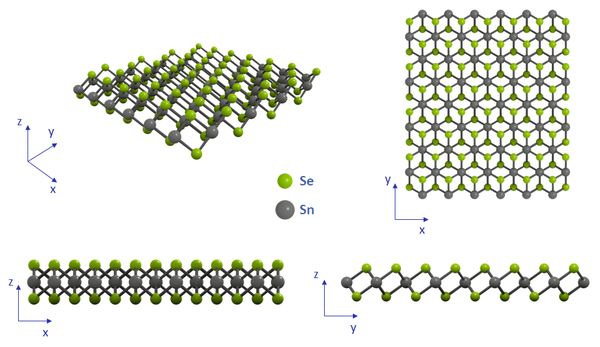
Structure of Tin(IV) Selenide
SnSe2 has been reported to have two different crystal structures: the 2H hexagonal phase, and the CdI2-type 1T phase. Currently, there is inconclusive evidence as to which phase is the most stable and frequently observed (2H-SnSe shown below). However, SnSe2 crystallizes in the CdI2-type lattice.
Like most of the transitional metal dichalogenides (TMDCs), it is composed of two-dimensional Se-Sn-Se sheets stacked on top of one another. Within each layer, every six selenium atoms are located at the corners of an octahedron, and feature an inversion symmetry (with respect to the central tin atom). The layered structure (bound by the weak Van der Waals forces) allows exfoliation in both solid and liquid forms to peel off layers from bulk crystals or powder.
Literature and Reviews
- Layer-dependent properties of SnS2 and SnSe2 novel two-dimensional materials, J. Gonzalez et al., Phys. Rev. B 94, 125443 (2016); DOI: 10.1103/PhysRevB.94.125443.
- SnSe2 field-effect transistors with high drive current, Y. Su et al., Appl. Phys. Lett., 103, 263104 (2013); doi: 10.1063/1.4857495.
- Temperature dependence of Raman shifts in layered ReSe2 and SnSe2 semiconductor nanosheets, A. Taube et al., Appl. Phys. Lett., 107, 013105 (2015); doi: 10.1063/1.4926508.
Related Products
We stock a wide range of 2D materials available to purchase online. Please contact us if you cannot find what you are looking for.



