Tantalum Disulfide (TaS2) Powder and Crystal
CAS Number 12143-72-5
2D Materials, Low Dimensional Materials, Materials, Transition Metal Chalcogenides (TMCs),Low price, high purity 2D metal tantalum disulfide powder and crystals
For the development of next-generation electronics, optoelectronics, and nanotechnology
Technical Data | MSDS | Structure | Literature and Reviews | Related Products | Resources and Support
Tantalum disulfide (TaS2), CAS number 12143-72-5, is a family member of two-dimensional (2D) metallic transition metal dichalcogenides (MTMDCs). It is of great research interest due to its rich phase diagrams that include superconductivity, charge-density wave (CDW), and metal-insulator transitions.
Tantalum disulfide crystal have stacked layers, in which sheets of transition metal atoms lie between the chalcogen atom sheets in an S-Ta-S sequence. Layered TaS2 exhibits a unique combination of valuable structural, mechanical, and electronic phases. 1T and 2H phases are the most representative structures.
Coexistence of CDW order and superconductivity has been found in atomically-thin TaS2. Such properties are mainly attributed to its reduced dimensionality and the induced quantum confinement effect. Reduced dimensionality can strengthen superconductivity in the case of thin TaS2, as opposed to the weakening effect that has been found in other 2D materials.
Bulk 2H-TaS2 exhibits a charge density wave (CDW) transition at 70K and a superconductivity transition at 0.8K [1].
We supply low price tantalum disulfide in several different forms for a range of applications.
High Purity
≥99.999% TaS2 Crystal
Worldwide shipping
Quick and reliable shipping
Low Cost
Low Cost TaS2
Powder & Crystal
Different Forms of Tantalum Disulfide

Tantalum Disulfide Powder
Can be used for preparation of tantalum disulfide nanoplates and ultrathin films
Sold by weight
≥99.995% purity
From £350

Tantalum Disulfide Crystals by Size
Can be used to produce single or few-layer tantalum disulfide sheets via mechanical or liquid exfoliation
Small (≥10 mm2) or medium (≥25 mm2) crystals available*
≥99.999% purity
From £520
*Typical representative size, areas/dimensions may vary
Bulk single tantalum disulfide crystal is most commonly used as sources from which single or few-layer sheets can be obtained via either mechanical or liquid exfoliation.
Tantalum disulfide powder can also be used to prepare TaS2 nanosheets and nanoparticles by liquid-exfoliation (normally assisted by sonication). Copper, lead, tin and lithium can be intercalated between the layers tantalum disulfide especially for superconducting transition temperature investigations.
Technical Data
| CAS Number | 12143-72-5 |
| Chemical Formula | TaS2 |
| Molecular Weight | 245.07 g/mol |
| Bandgap | N/A |
| Preparation | Synthetic - Chemical Vapor Transport (CVT) |
| Structure | Hexagonal (2H) |
| Electronic Properties | 2D CDW materials |
| Melting Point | N/A |
| Color | Dark brown |
| Synonyms | Dithioxotantalum, Tantalum(IV) sulfide, Bis(sulfanylidene)tantalum |
| Classification / Family | Transition metal dichalcogenides (TMDCs), 2D charge density wave (CDW) materials, Nano-electronics, Nano-photonics, Superconductivity, Photovoltaic, Materials science |
Product Details
| Form | Purity |
|---|---|
| Powder | ≥99.995% |
| Crystal | ≥99.999% |
Pricing Table
| Product Code | Form | Size/Weight* | Price |
|---|---|---|---|
| M2144C1 | Powder | 1 g | £350 |
| M2144A10 | Crystal | Small (≥10 mm2) | £520 ea. |
| M2144A25 | Crystal | Medium (≥25 mm2) | £850 ea. |
*typical representative size, areas/dimensions may vary
Shipping is free for qualifying orders.
MSDS Documents
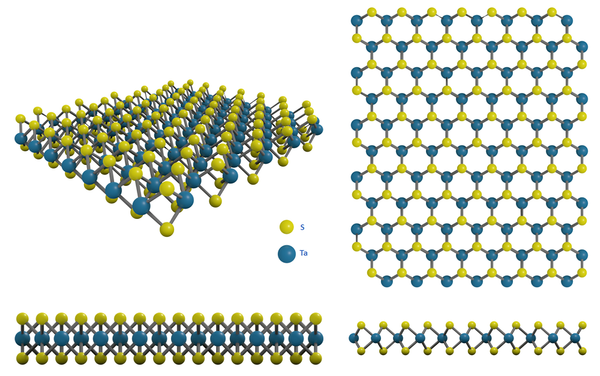
Structure of Tantalum Disulfide
Tantalum disulfide (TaS2) crystal has stacked layers, in which sheets of transition metal atoms lie between the chalcogen atom sheets in an S-Ta-S sequence. In each sheet, atoms are closely packed in a hexagonal pattern. The chemical bonding within the layer is covalent, while individual layers are bound by van der Waals (vdWs). This makes intercalation between layers possible and allows layers to be peeled off by exfoliation.
Layered TaS2 exhibits a unique combination of valuable structural, mechanical, and electronic phases. 1T and 2H phases are the most representative structures. The distinction between two phases can be the S-Ta-S coordinated situations, which are recognized as octahedral coordination (1T) and prismatic coordination (2H).
Literature and Reviews
- Enhanced superconductivity in atomically thin TaS2, E. Navarro-Moratalla et al., Nat. Commun., 7:11043 (2016); DOI: 10.1038/ncomms11043.
- Enhanced superconductivity upon weakening of charge density wave transport in 2H-TaS2 in the two-dimensional limit, Y. Yang et al., Phys. Rev., 98, 035203 (2018); DOI: 10.1103/PhysRevB.98.035203.
- Toward Exploring the Structure of Monolayer to Few-layer TaS2 by Efficient Ultrasound-free Exfoliation, Y. Hu et al., Nanoscale Res. Lett., 13:20 (2018); DOI 10.1186/s11671-018-2439-z.
Related Products
We stock a wide range of 2D materials available to purchase online. Please contact us if you cannot find what you are looking for.



