Molybdenum Tungsten Disulfide (MoWS2) Powder
CAS Number 109657-36-5
2D Materials, Low Dimensional Materials, Materials, Transition Metal Chalcogenides (TMCs),MoWS2 high purity powder
Used to prepare quantum dot solutions and nano-platelets
Technical Data | MSDS | Structure | Literature and Reviews | Related Products | Resources and Support
Molybdenum tungsten disulfide (MoWS2), CAS number 109657-36-5, is a transition metal dichalcogenide (TMDC) alloy. Alloying 2D TMDCs is an effective way of practically modulating the band gap. This is because alloys have good thermodynamic stability at room temperature.
High Purity
MoWS2 high purity powder
Worldwide Shipping
Quick and reliable shipping
Low Cost
Low Cost Molybdenum Tungsten Disulfide Powder
Applications
Used to prepare quantum dot solutions and nano-platelets
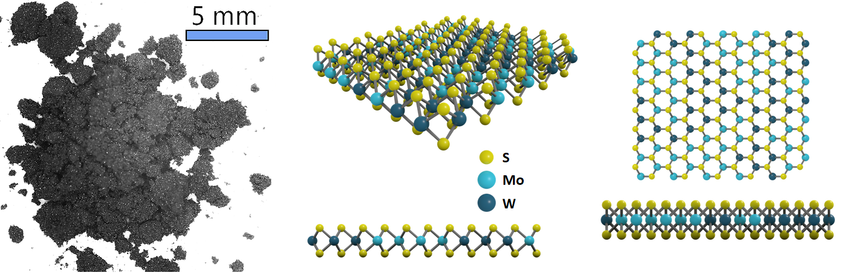
Like molybdenum disulfide powder and tungsten disulfide powder, MoWS2 powder has a layered hexagonal crystal structure. The bulk alloys are formed by stacking monolayer alloy together via van der Waals interactions. Each monolayer contains one MoW plane sandwiched by two S planes, represented as an S-MoW-S layer.
Technical Data
| CAS Number | 109657-36-5 |
| Chemical Formula | MoWS2 |
| Molecular Weight | 204.01 g/mol |
| Bandgap | ~1.90 eV [1] |
| Preparation | Synthetic - Chemical Vapor Transport (CVT) |
| Structure | Hexagonal |
| Electronic Properties | 2D Semiconductor |
| Melting Point | N/A |
| Color | Black |
| Classification / Family | Transition metal dichalcogenides (TMDCs) alloy, 2D semiconductor materials, Nano-electronics, Nano-photonics, Materials science |
Product Details
| Form | Purity |
|---|---|
| Powder | ≥99.995% |
Pricing Table
| Product Code | Form | Quantity | Price |
|---|---|---|---|
| M2141C1 | Powder | 500 mg | £220 |
| M2141C1 | Powder | 1 g | £360 |
MSDS Document
Molybdenum tungsten disulfide powder
Structure of Molybdenum Disulfide Powder
Literature and Reviews
- Towards band structure and band offset engineering of monolayer Mo(1−x)W(x)S2 via Strain, J.-S. Kim et al, 2D Mater. 5, 015008 (2018); doi: 10.1088/2053-1583/aa8e71.
- Experimental and First-Principles Investigation of MoWS2 with High Hydrogen Evolution Performance, H. Li et al., ACS Appl. Mater. Interfaces 2016, 8, 29442−29451; DOI: 10.1021/acsami.6b09620.
- Ordered and Disordered Phases in Mo1−xWxS2 Monolayer, W. Tan et al., Sci. Rep., 7:15124 (2017); DOI:10.1038/s41598-017-15286-9.
Related Products
We stock a wide range of 2D materials available to purchase online. Please contact us if you cannot find what you are looking for.



