Tungsten Disulfide (WS2) Powder
CAS Number 12138-09-9
2D Materials, Low Dimensional Materials, Materials, Transition Metal Chalcogenides (TMCs),High purity tungsten disulfide powder
For applications as a high performance lubricant
Technical Data | MSDS | Structure | Literature and Reviews | Related Products | Resources and Support
One of the most distinctive features of tungsten disulfide powder (WS2, CAS number 12138-09-9) is its performance as a lubricant, even under harsh conditions (e.g. high temperature and high pressure), its coefficient of friction (CoF) of 0.03 is nearly unmatched.
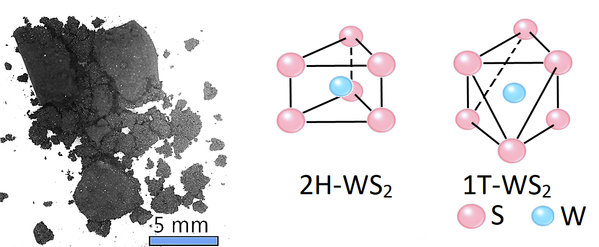
Depending on the techniques used in its preparation, exfoliated 2D WS2 layers have three crystal phases: octahedral (1T) and hexagonal (2H and 3R). Such phases provide opportunities for structure engineering at an atomic level to develop new optoelectronic properties. It is known that the 2H (antiparallel) and 3R (parallel) hexagonal phases are semiconducting.
Of the two hexagonal phases, 2H is the more stable form. The octahedrally-coordinated 1T phase is not stable in a bulk form, and is associated solely with single-layer films. It is believed that the 1T phase is metallic and can be transformed from 2H WS2 by electron doping (e.g. by electron irradiation or lithium ion intercalation).
High Purity
>99% Tungsten Disulfide Purity
Worldwide shipping
Quick and reliable shipping
Low Cost
Low Cost Tungsten Disulfide
Applications
Applications as a high performance lubricant
Technical Data
| CAS Number | 12138-09-9 |
| Chemical Formula | WS2 |
| Molecular Weight | 247.97 g/mol |
| Bandgap | 1.4 - 2.01 eV [1] |
| Preparation | Synthetic - Chemical Vapor Transport (CVT) |
| Structure | Hexagonal (2H) |
| Electronic Properties | 2D semiconductor |
| Melting Point | 1250 °C (lit.) |
| Color | Black |
| Synonyms | Tungsten sulfide, Tungsten sulfide, Tungsten(IV) sulfide |
| Classification / Family | Transition metal dichalcogenides (TMDCs), 2D semiconductor materials, NIR band-gap, Nano-electronics, Nano-photonics, Transistors, Photovoltaics, Materials science |
Product Details
| Form | Purity |
|---|---|
| Powder | ≥99.995% |
Pricing Table
| Product Code | Form | Quantity | Price |
|---|---|---|---|
| M2110C1 | Powder | 500 mg | £220 |
| M2110C1 | Powder | 1 g | £350 |
MSDS Document
Structure of Tungsten Disulfide Powder
Literature and Reviews
- Controlled Synthesis and Transfer of Large-Area WS2 Sheets: From Single Layer to Few Layers, A. Elı´as et al., ACS Nano, 7 (6), 5235–5242 (2013); DOI: 10.1021/nn400971k.
- An effective liquid-phase exfoliation approach to fabricate tungsten disulfide into ultrathin two dimensional semiconducting nanosheets, R. Jha et al., J. Mater. Sci., 52:7256–7268 (2017); DOI 10.1007/s10853-017-0962-4.
- Enhanced Catalytic Activities of Surfactant Assisted Exfoliated WS2 Nanodots for Hydrogen Evolution, X Zhao et al., ACS Nano 2016, 10, 2159−2166; DOI: 10.1021/acsnano.5b06653.
Related Products
We stock a wide range of 2D materials available to purchase online. Please contact us if you cannot find what you are looking for.



