Graphene Oxide Powder
CAS Number 2640657-49-2
2D Materials, Graphene Materials, Low Dimensional Materials, Materials2D Material for Processing at High Concentrations
High quality and high purity (99.5%) single layer graphene oxide
Overview | Product Information | Related Products

Graphene oxide (CAS number 2640657-49-2) is one of the most popular 2D materials available. This is due to the wide range of fields that it can be applied to. It has a distinct advantage over other 2D materials (such as graphene), as it is easily dispersed within solution; allowing for processing at high concentrations. This has opened it up for use in applications such as optical coatings, transparent conductors, thin-film batteries, chemical resistant coatings, water purification, and many more.
Ossila have two types of graphene oxide powders available, with flake sizes between 1 - 5 µm.
High Purity
99.5% purity graphene oxide
Worldwide Shipping
Quick and reliable shipping
Low Price
Affordable graphene oxide powders
Easy Dispersion
For high concentrations
General Information
| CAS number | 2640657-49-2 |
|---|---|
| Chemical formula | CxHyOz |
| Recommended Solvents | H2O, DMF, IPA |
| Synonyms | Single layer GO, Few-layer GO, GO |
| Classification or Family | 2D semiconducting materials, Carbon nanomaterials, Graphene, Organic electronics |
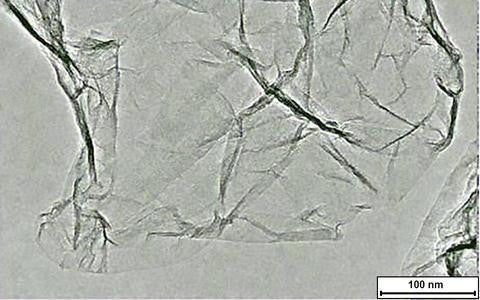
Single-Layer Graphene Oxide Powders
| Product Code | M0880A1 |
|---|---|
| Flake Size | ~ 2 μm |
| Flake Thickness | ~ 1 nm |
| Single Layer Ratio | >99% |
| Purity | >99.5% |
| Appearance | Black/Brown Sheets/Powder |
Few-Layer Graphene Oxide Powders
| Product Code | M0880A2 |
|---|---|
| Average Number of Graphene Layers | 2 – 5 layers |
| Flake Size/Diameter | 4.5 µm |
| Surface Area | 420 m2/g |
| Purity | 99.8% |
| Appearance | Dark grey/brown powder |
MSDS Documents
Graphene oxide powders MSDS Sheet
Pricing Table
| Batch | Quantity | Price |
|---|---|---|
| M0880A1 | 250 mg | £240 |
| M0880A1 | 500 mg | £380 |
| M0880A2 | 250 mg | £90 |
| M0880A2 | 500 mg | £140 |
| M0880A2 | 1 g | £220 |
| M0880A2 | 2 g | £355 |

Dispersion Guide
Due to the presence of oxygen and hydroxide groups, the dispersibility of this material is significantly better than other 2D materials (such as graphene). High concentrations of GO can be dispersed in polar solvents, such as water. At Ossila, we have found that the most stable solutions can be produced using the following recipe:
- Weigh out desired amount of material, this can go up to at least 5 mg.ml-1.
- Add 1:1 ratio of deionized water to isopropyl alcohol.
- Shake vigorously to break up material.
- A short treatment in an ultrasonic bath will rapidly disperse the material.
- For larger flakes, use a mechanical agitator instead (as sonication may damage the flakes).
References
- An improved Hummers method for eco-friendly synthesis of graphene oxide, J. Chen et al., Carbon 64, 225-229 (2013); http://dx.doi.org/10.1016/j.carbon.2013.07.055.
- Synthesis of few-layered, high-purity graphene oxide sheets from different graphite sources for biology, D. A. Jasim et al., 2D Mater. 3, 014006 (2016); doi:10.1088/2053-1583/3/1/014006.
- Preparation and Characterization of Graphene Oxide, J. Song et al., J. Nanomater., 276143 (2014); http://dx.doi.org/10.1155/2014/276143.
Related Products
We stock a wide range of 2D materials available to purchase online. Please contact us if you cannot find what you are looking for.



