Tin(II) Selenide (SnSe) Crystal
CAS Number 1315-06-6
2D Materials, Low Dimensional Materials, Materials, Post-Transition Metal Chalcogenides (PTMCs),Tin(II) selenide crystal, high purity narrow band gap semiconductor
Comprised of environmentally friendly elements
Technical Data | MSDS | Structure | Literature and Reviews | Related Products | Resources and Support
Tin(II) selenide (SnSe), CAS number 1315-06-6, is a narrow band gap semiconductor comprised of environmentally friendly and earth abundant elements.
SnSe has recently proven to be an extraordinarily promising thermoelectric material with intrinsically ultra-low lattice thermal conductivity and a record figure of merit up to 2.6 at a higher temperature (813 K). This enables direct and reversible conversion between thermal and electrical energy, and provides a viable route for power generation from waste heat.
With an indirect bandgap of 1.30 eV and direct bandgap of 1.21 eV, SnSe has great potential to be utilized as an efficient material for solar energy conversion.
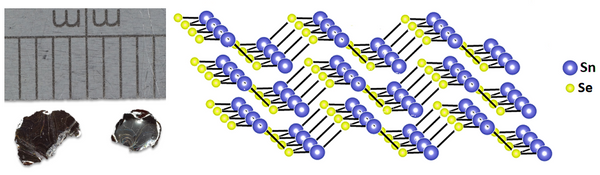
SnSe belongs to the class of layered semiconductors. It is made up of tightly bound layers formed by double planes each of which consists of zigzag chains of tin and selenium atoms. At room temperature, SnSe adopts orthorhombic crystal structure which can be compared to that of black phosphorus.
High Purity
≥99.999% Crystal Purity
Worldwide shipping
Quick and reliable shipping
Semiconductor
Narrow band gap semiconductor
Environmentally friendly
Comprised of environmentally friendly elements
Technical Data
| CAS Number | 1315-06-6 |
| Chemical Formula | SnSe |
| Molecular Weight | 197.67 g/mol |
| Bandgap | 0.91 - 1.79 eV [1] |
| Preparation | Synthetic - Chemical Vapor Transport (CVT) |
| Structure | Orthorhombic |
| Electronic Properties | 2D semiconductor |
| Melting Point | 861 °C (lit.) |
| Color | Metallic |
| Synonyms | Stannous selenide, Tin selenide |
| Classification / Family | Transition metal dichalcogenides (TMDCs), 2D semiconductor materials, Nano-electronics, Nano-photonics, Materials science |
Product Details
| Form | Purity |
|---|---|
| Crystal | ≥99.999% |
Pricing Table
| Product Code | Form | Size* | Quantity (EA) | Price |
|---|---|---|---|---|
| M2116A10 | Crystal | Small (≥10 mm2) | 1 | £520 |
| M2116A25 | Crystal | Medium (≥25 mm2) | 1 | £850 |
*Typical representative size, areas/dimensions may vary
Shipping is free for qualifying orders.
MSDS Documents
Structure of Tin(II) Selenide Crystal
Literature and Reviews
- Liquid Exfoliation Few-Layer SnSe Nanosheets with Tunable Band Gap, Y. Huang et al., J. Phys. Chem. C, 121 (32), 17530–17537 (2017); DOI: 10.1021/acs.jpcc.7b06096.
- Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals, L. Zhao et al., Nature, 508, 373-377 (2014); doi: 10.1038/nature13184.
- Quasiparticle band structures and thermoelectric transport properties of p-type SnSe, G. Shi et al., J. Appl. Phys., 117, 065103 (2015); doi: 10.1063/1.4907805.
Related Products
We stock a wide range of 2D materials available to purchase online. Please contact us if you cannot find what you are looking for.



