4,4'-((4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)azanediyl)dibenzaldehyde
CAS Number 2009169-65-5
Boronates, Carbaldehyde Monomers, Chemistry Building Blocks, COF Ligands, Materials, Monomers,Covalent organic frameworks (COFs) triphenylamine ligand
For the synthesis of COFs for AIE molecules, metal-COF complexes and dyes in DSSCs
Specifications | MSDS | Literature and Reviews
4,4'-((4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)azanediyl)dibenzaldehyde (CAS number 2009169-65-5 ), has a triphenylamine core featuring a pinacolborane and two aldehyde groups. The pinacolborane group enables the Suzuki coupling reaction while the aldehyde groups can undergo aldol reaction and amination reaction. 4,4'-((4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)azanediyl)dibenzaldehyde is employed to synthesize aggregation-induced emission molecules (TPA-AN-TPM). In the synthesis, 4,4'-((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)azanediyl)dibenzaldehyde acts as a linker for connecting dimethylpyranyldene-malononitrile and anthracenes. The resulting molecule forms ultrabright red AIE dots with absolute quantum yield of 12.9%.
4,4'-((4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)azanediyl)dibenzaldehyde is also utilized in the preparation of dyes with thiazole. This triphenylamine linked bisthiazole based dye is applied in dye-sensitized solar cells (DSSCs), displaying current density of 13.60 mA/cm-2, fill factor of 58.2%, open-circuit voltage of 620 mV and power conversion efficiency (PCE) of 4.94%.
Duo functionality
Enabling post-functionalization
High Purity
>98% Purity
Worldwide shipping
Quick and reliable shipping
MOF and COF ligands
Aldehyde and Bpin ligand for cross-linked COF networks
General Information
| CAS Number | 2009169-65-5 |
| Chemical Formula | C26H26BNO4 |
| Full Name | 4,4'-{[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]imino}dibenzaldehyde |
| Molecular Weight | 427.30 g/mol |
| Synonyms | N/A |
| Classification / Family | COFs, Aldehyde ligands, Pinacolborane ligands, AIEs, Metal-COF complexes, Dyes |
Chemical Structure
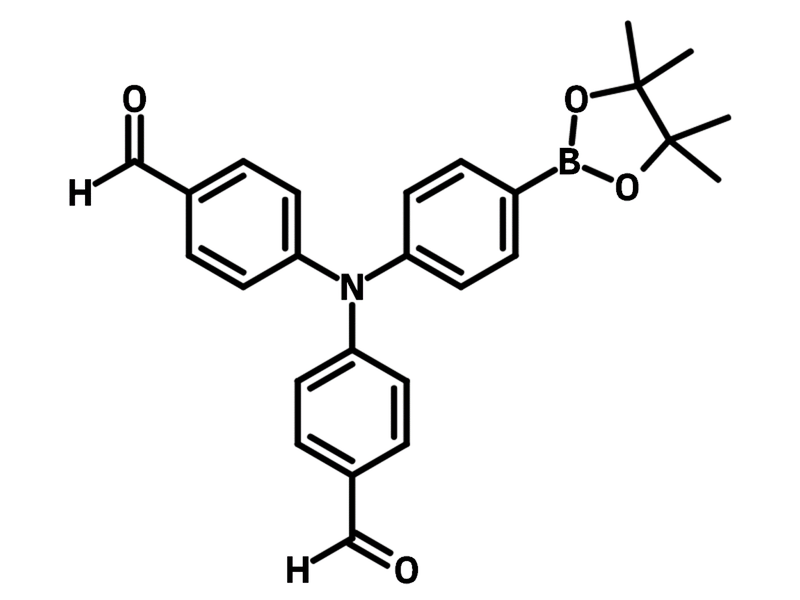
Product Details
| Purity | 98% |
| Melting Point | N/A |
| Appearance | Yellow to orange powder |
MSDS Documentation
4,4'-((4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)azanediyl)dibenzaldehyde MSDS Sheet
Literature and Reviews
- Conjugated and nonconjugated bipolar-transporting dinuclear europium(III) complexes involving triphenylamine and oxadiazole units: synthesis, photophysical and electroluminescent properties, Y. Liu et al., Tetrahedron, 69 (23), 4679–4686 (2013); DOI: 10.1016/j.tet.2013.03.092.
- Enhanced photovoltaic performance using biomass derived nano 3D ZnO hierarchical superstructures and a D−A type CS-Symmetric triphenylamine linked bisthiazole, Electrochim. Acta, M. Ansari et al., 259, 262–275 (2018); DOI: 10.1016/j.electacta.2017.10.174.
- Cs-symmetric triphenylamine-linked bisthiazole-based metal-free donor–acceptor organic dye for efficient ZnO nanoparticles-based dye-sensitized solar cells: synthesis, theoretical studies, and photovoltaic properties, R. Maragani et al., ACS Omega, 2(9), 5981–5991 (2017); DOI: 10.1021/acsomega.7b01100.
