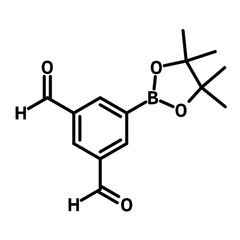
5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)isophthalaldehyde
CAS Number 945865-80-5
Chemistry Building Blocks, COF Ligands, Materials, Porous Organic FrameworksCovalent organic frameworks (COFs) isophthalaldehyde ligand
a versatile ligand used for synthesizing COFs and macromolecules with applications in iodine capture, OLEDs and light induced hydrogen generation
Specifications | MSDS | Literature and Reviews
5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)isophthalaldehyde (CAS number 945865-80-5) is an isophthalaldehyde that contains a pinacolborate group (tetramethyl dioxaboranyl, Bpin). With two aldehyde groups, 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)isophthalaldehyde can crosslink with amines for producing COFs with imine linkages. The Bpin group allows for its combination with other functional molecules such as pyrenes through the Suzuki-Miyaura cross-coupling reaction. The resulting pyrene-isophthalaldehyde reacts with cyclohexanediamine forming a COF cage, which is studied for its photocatalytic reaction of converting benzeneboronic acid to phenol with a 99% conversion. The functionalities of the COFs cages are versatile and dependent on the specific functional molecules employed in the reaction. For instance, the inclusion of bipyridine provides the COFs cage with the ability to capture iodine (up to 5.8 g/g).
Benzimidazole-triazine based materials for exciplex-OLEDs can be prepared from 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)isophthalaldehyde, triazine derivatives and N-phenyl-o-phenylenediamine. The exciplex-OLEDs exhibit luminous efficacy of 80.4 lm/W, current efficiency of 60.1 cd/A and external quantum efficiency of 18.4%.
Duo functionality
Enabling post-functionalization
High Purity
>98% Purity
Worldwide shipping
Quick and reliable shipping
MOF and COF ligands
Aldehyde and Bpin ligand for cross-linked COF networks
General Information
| CAS Number | 945865-80-5 |
| Chemical Formula | C14H17BO4 |
| Full Name | 5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)isophthalaldehyde |
| Molecular Weight | 260.09 g/mol |
| Synonyms | 5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzene-1,3-dicarbaldehyde |
| Classification / Family | Isophthalaldehydes, Dioxaborolanes, COFs, Macromolecules, Iodine capture, OLEDs, Hydrogen evolution, Photocatalysts |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | N/A |
| Appearance | White to off-White powder |
MSDS Documentation
5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)isophthalaldehyde MSDS Sheet
Literature and Reviews
- Tuning molecular chromophores of isoreticular covalent organic frameworks for visible light-induced hydrogen generation, W. Li et al., Adv. Funct. Mater., 32, 2207394(2022); DOI: 10.1002/adfm.202207394.
- Stimuli-responsive porous molecular crystal with reversible modulation of porosity, N. Sun et al., ACS Appl. Mater. Interfaces, 14(1), 1519–1525(2021); DOI: 10.1021/acsami.1c18368.
- Benzimidazole-triazine based exciplex films as emitters and hosts to construct highly efficient OLEDs with small efficiency roll-off, B. Liang et al., J. Mater. Chem. C, 8, 2700–2708(2020); DOI: 10.1039/C9TC06212B.
