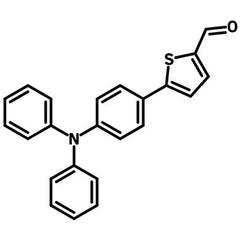
5-(4-(Diphenylamino)phenyl)thiophene-2-carbaldehyde
CAS Number 291279-14-6
Carbaldehyde Monomers, Chemistry Building Blocks, COF Ligands, Materials, Monomers, Porous Organic FrameworksCovalent Organic Frameworks (COFs) Triphenylamine Ligand
A formyl functional ligand linker for COFs in application of aggregation induced emission (AIE) two-photon fluorescent probe
Specifications | MSDS | Literature and Reviews
5-(4-(diphenylamino)phenyl)thiophene-2-carbaldehyde (DPAPTA), CAS number 291279-14-6, is consisted of a triphenylamine core with a 2-carbaldehydethiophene attached to one of the phenyl rings. The aldehyde functional group is ideal to form double bond π-bridges with amines via condensation reaction.
Derived from 5-(4-(diphenylamino)phenyl)thiophene-2-carbaldehyde, aggregation induced emission (AIE) two-photon fluorescent probe AIETP demonstrated efficient brain vasculature imaging with high brightness and improved photostability. AIETP NPs showed excellent two-photon cross section and in vivo stability apart from demonstrating great cell permeability and biocompatibility.
A mitochondria-anchoring photosensitizer, TTVPHE, also derived from 5-(4-(diphenylamino)phenyl)thiophene-2-carbaldehyde with AIE characteristics for efficient ablation of extrahepatic cholangiocarcinoma cells. The TTVPHE probe showed great advantage for fast cell penetration and selective mitochondria-targeting abilities, and excellent reactive oxygen species (ROS) generation under the visible light irradiation.
Triphenyl amine-thiophene conjugated with benzothiazole derivative fluorescent probe TP-1, showed a visible color change from dark yellow to colorless and subsequently the fluorescence intensity increase after the addition of the cyanide anions in the linear range of 1 – 100 μM. The limit of detection for cyanide ions by the probe TP-1 was found to be 4.24 x 10−8 M.
TTVP, a D–A type compound comprising triphenylamine and thiophene fragments (D and π-bridge) and a carbon–carbon double bond showed AIE features, good water-solubility and intense emission in the NIR region. This AIE luminogen (AIEgen) can specifically light up the cell membrane without the involvement of a washing procedure. The staining process is ultrafast and easy-to-operate, performed by simply shaking the culture with cells at room temperature for only a few seconds after the addition of the AIEgen.
MOF and COF ligands
Aldehyde ligand for end-capping COF networks
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
Facile reactions
Aldehyde possesses excellent reactivity
General Information
| CAS Number | 291279-14-6 |
|---|---|
| Chemical Formula | C23H17NOS |
| Full Name | 5-(4-(Diphenylamino)phenyl)thiophene-2-carbaldehyde |
| Molecular Weight | 355.45 g/mol |
| Synonyms | DPAPTA, 2-Thiophenecarboxaldehyde, 5-[4-(diphenylamino)phenyl]- |
| Classification / Family | Triphenylamine, COF ligands, Aggregation induced emission (AIE) |
Chemical Structure
Product Details
| Purity | >98% |
|---|---|
| Melting Point | N/A |
| Appearance | Yellow to orange powder/crystals |
MSDS Documentation
5-(4-(Diphenylamino)phenyl)thiophene-2-carbaldehyde MSDS Sheet
Literature and Reviews
-
AIE-active two-photon fluorescent nanoprobe with NIR-II light excitability for highly efficient deep brain vasculature imaging, S. Samanta et al., Theranostics, 11(5), 2137–2148 (2021); DOI: 10.7150/thno.53780.
-
Mitochondria-anchoring and AIE-active photosensitizer for self-monitored cholangiocarcinoma therapy, T. Zhou et al., Mater. Chem. Front., 4, 3201-3208 (2020); DOI: 10.1039/D0QM00503G.
-
Tuning the HOMO and LUMO Energy Levels of Organic Chromophores for Dye Sensitized Solar Cells, D. Hagberg et al., J. Org. Chem., 72 (25), 9550–9556 (2007); DOI: 10.1021/jo701592x.
