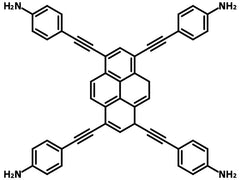
4,4',4'',4'''-(Pyrene-1,3,6,8-tetrayltetrakis(ethyne-2,1-diyl))tetraaniline
CAS Number 1404196-75-3
Chemistry Building Blocks, COF Ligands, Materials, Porous Organic FrameworksCovalent Organic Frameworks (COFs) Pyrene Ligand
A tectonic bridging ligand linker for COFs in applications of effective photo-reductive dehalogenation of phenacyl bromide derivatives and enantio-selective α-alkylation of aldehydes
Specifications | MSDS | Literature and Reviews
4,4',4'',4'''-(Pyrene-1,3,6,8-tetrayltetrakis(ethyne-2,1-diyl))tetraaniline (TAEPy), CAS number 1404196-75-3, contains a pyrene core with four 4-ethynylaniline side arms at 1,3,6,8-positions. 1,3,6,8-tetrakis(4-aminophenyl)pyrene is mainly investigated in covalent organic frameworks (COFs) for its superb properties such as large surface area, high crystallinity, excellent stability, and good photoelectric activity.
Covalent organic framework COF-JLU22, prepared from TAEPy and an electron-deficient linker 4,7-formyldiphenylbenzo[c][1,2,5]thiadiazole (FDPBT), possessed robust thermal, chemical and photochemical stability, apart from its large pore volume and high crystallinity. Being an electron donor–acceptor type COF, JLU22 is relatively red-shifted in absorption wavelength to enhance the photocatalytic activity of the resulting COF. COF-JLU22 is highly efficient for dehalogenation of phenacyl bromide derivatives and effectively photo-catalyze the enantio-selective α-alkylation of aldehydes.
MOF and COF ligands
Amine ligand for cross-linked COF/MOF networks
Worldwide shipping
Quick and reliable shipping
High purity
>97% High purity
Facile reactions
Amine possesses excellent reactivity
General Information
| CAS Number | 1404196-75-3 |
|---|---|
| Chemical Formula | C48H30N4 |
| Full Name | 4,4',4'',4'''-(Pyrene-1,3,6,8-tetrayltetrakis(ethyne-2,1-diyl))tetraaniline |
| Molecular Weight | 662.78 g/mol |
| Synonyms | TAEPy, TAPE, 1,3,6,8-Tetrakis((4-aminophenyl)ethynyl)pyrene |
| Classification / Family | Pyrenes, Tetraanilines, COF ligands |
Chemical Structure
Product Details
| Purity | >97% |
|---|---|
| Melting Point | N/A |
| Appearance | Brown to orange to red powder/crystals |
MSDS Documentation
4,4',4'',4'''-(Pyrene-1,3,6,8-tetrayltetrakis(ethyne-2,1-diyl))tetraaniline MSDS Sheet
Literature and Reviews
-
De Novo Design and Facile Synthesis of 2D Covalent Organic Frameworks: A Two-in-One Strategy, Y. Li et al., J. Am. Chem. Soc., 141 (35), 13822–13828 (2019); DOI: 10.1021/jacs.9b03463.
-
Recent Advances on Conductive 2D Covalent Organic Frameworks, G. Bian et al., Small, 17 (22), 2006043 (2021); DOI: 10.1002/smll.202006043.
- Constructing Stable Chromenoquinoline-Based Covalent Organic Frameworks via Intramolecular Povarov Reaction, X. Ren et al., J. Am. Chem. Soc., 144 (6), 2488–2494 (2022); DOI: 10.1021/jacs.1c13005.
