1,1,2,2-Tetrakis(4-ethynylphenyl)ethene
CAS Number 4863-90-5
Chemistry Building Blocks, COF Ligands, Materials, Porous Organic FrameworksCovalent Organic Frameworks (COFs) Ethene Ligand
A symmetrical butterfly shaped bridging ligand linker for covalent organic frameworks (COFs) in application of chemosensors of explosives, carbon dioxide adsorption and hydrogen evolution reactions (HERs)
Specifications | MSDS | Literature and Reviews
1,1,2,2-tetrakis(4-ethynylphenyl)ethene (TEPE) has four 4-ethynylphenyls that are joined by an ethene double bond. Thanks to their aggregation induced emission (AIE) characteristics though none-emissive at lower concentrations, tetraphenylethenes (TPEs) have been found to serve as chemosensors, stimuli-responsive nanomaterials, and active layers of efficient OLEDs.
Hyperbranched polyTPE with high molecular weight, high thermal stability and good solubility can be synthesized via homopolycyclotrimerization of 1,1,2,2-tetrakis(4-ethynylphenyl)ethene. The homopolymer can serve as a fluorescent chemosensor for the detection of explosives with a super amplification effect and large quenching constant up to 758,000 M−1. Conjugated organic polymer (PQTEE-COP) containing phenanthrenequinone redox centers and 1,1,2,2-tetrakis(4-ethynylphenyl)ethene linkers shows efficient photocatalytic H2O2 production of 3009 μmol g-1h−1 from H2O and O2 under visible light (λ ≥ 400 nm) irradiation without the use of any additional photosensitizers, organic scavengers or co-catalysts. Efficient charge separation and migration can be achieved by the phenanthrenequinone moieties accepting photo-induced electrons from electronic-rich tetrakis(4-ethynylphenyl)ethene moieties under visible light irradiation.
MOF and COF ligands
Ethene ligand for cross-linked COF networks
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
Facile reactions
Readily for homopolycyclotrimerization
P[5]-TPE-CMP, a luminescent conjugated macrocycle polymer (CMP) with strong two-photon fluorescence property derived from pillar[5]arene and a 1,1,2,2-tetrakis(4-ethynylphenyl)ethylene shows great stability against photobleaching, P[5]-TPE-CMP also exhibits highly selective cation sensing capability toward Fe3+ at different excitation wavelengths (both UV and red–near-infrared regions) and carcinogenic organic dye 4-amino azobenzene. FCMP-600@4, a covalent organic framework between fluorine rich monomer 4,4′-dibromooctafluorobiphenyl (DBFB) and 1,1,2,2-tetrakis(4-ethynylphenyl)ethene, shows considerable adsorption capacity of CO2 of 5.35 mmol g−1 at 273 K, and 4.18 mmol g−1 at 298 K, higher than the reported values for most porous polymers.
Imine bridged covalent organic framework TEPE-Im show high antibacterial activity at 15 mg mL−1. TPET-Im COFs display the high specific capacitance of 63 F g−1, coupled with an energy density of 8.73 W h Kg−1 in super capacitive studies.
General Information
| CAS Number | 4863-90-5 |
|---|---|
| Chemical Formula | C34H20 |
| Full Name | 1,1,2,2-Tetrakis(4-ethynylphenyl)ethene |
| Molecular Weight | 428.53 g/mol |
| Synonyms | TEPE, TEE, TPE, TETPE, TPET, Tetrakis(4-ethynylphenyl)ethene |
| Classification / Family | Ethenes, Ethynyls, COF ligands |
Chemical Structure
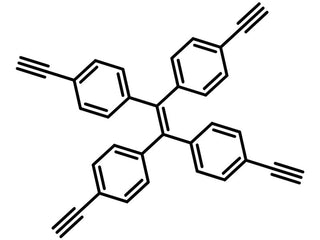
Product Details
| Purity | > 98% |
|---|---|
| Melting Point | Tm = 155.5 °C (lit.) |
| Appearance | Beige to light brown powder powder/crystals |
MSDS Documentation
1,1,2,2-Tetrakis(4-ethynylphenyl)ethene MSDS Sheet
Literature and Reviews
-
Ethynyl-Capped Hyperbranched Conjugated Polytriazole: Click Polymerization, Clickable Modification, and Aggregation-Enhanced Emission, J. Wang et al., Macromolecules, 45, 7692−770 (2012); DOI: 10.1021/ma3017037.
-
Homopolycyclotrimerization of A4-type tetrayne: A new approach for the creation of a soluble hyperbranched poly(tetraphenylethene) with multifunctionalities, R. Hu et al., J. Polym. Sci., Part A: Polym. Chem., 51, 4752–4764 (2013); DOI: 10.1002/pola.26897.
-
The construction of conjugated organic polymers containing phenanthrenequinone redox centers for visible-light-driven H2O2 production from H2O and O2 without any additives, X. Xu et al., Chem. Eng. J., 454, 139929 (2023); DOI: 10.1016/j.cej.2022.139929.
-
Tetraphenylethylene-Interweaving Conjugated Macrocycle Polymer Materials as Two-Photon Fluorescence Sensors for Metal Ions and Organic Molecules, X. Li et al., Adv. Mater., 30 (20), 1800177 (2018); DOI: 10.1002/adma.201800177.
- Synthesis of tunable porosity of fluorine-enriched porous organic polymer materials with excellent CO2, CH4 and iodine adsorption, G. Li et al., Sci. Rep., 7, 13972 (2017); DOI: 10.1038/s41598-017-14598-0.
