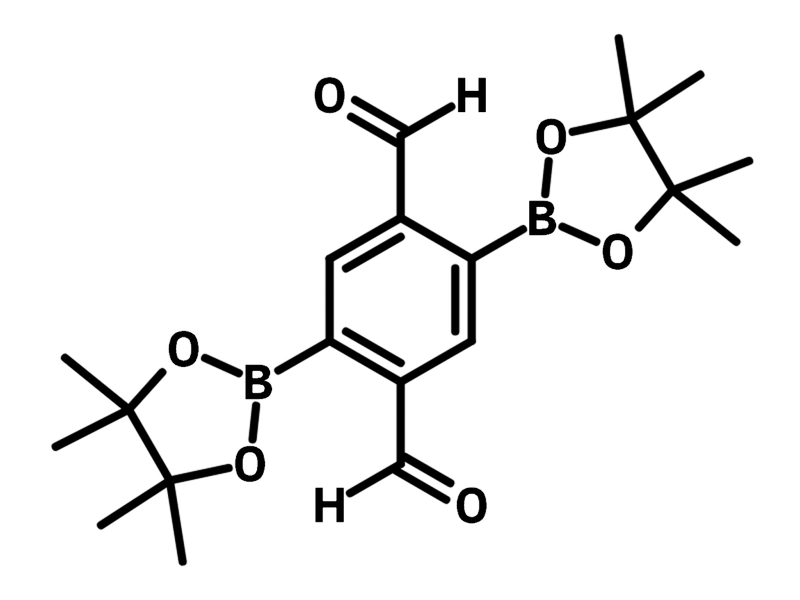
2,5-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)terephthalaldehyde
CAS Number 2305615-53-4
Boronates, Carbaldehyde Monomers, Chemistry Building Blocks, COF Ligands,Covalent organic frameworks (COFs) benzenedicarbaldehyde ligand
For the preparation of COFs used as porous materials and semiconducting polymers
Specifications | MSDS | Literature and Reviews
2,5-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)terephthalaldehyde (CAS number 2305615-53-4), is a benzene derivative with two aldehyde and two pinacolborane substituents. Both the aldehyde groups and pinacolborane groups are located at the para-positions. 2,5-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)terephthalaldehyde serves as a versatile ligand and it is readily employed as a monomer for Suzuki cross-coupling reactions and amination reactions. Notably, the functional groups do not interfere with each other during these reactions. It makes the COFs synthesized from 2,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)terephthalaldehyde ideal for post-functionalization after the polymer network is formed.
2,5-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)terephthalaldehyde is also used in the synthesis of poly(phenylenevinylene) for 2D semiconducting materials, through aldol condensation.
Duo functionality
Enabling post-functionalization
High Purity
>98% Purity
Worldwide shipping
Quick and reliable shipping
MOF and COF ligands
Aldehyde and Bpin ligand for cross-linked COF networks
General Information
| CAS Number | 2305615-53-4 |
| Chemical Formula | C20H28B2O6 |
| Full Name | 2,5-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)terephthalaldehyde |
| Molecular Weight | 386.05 g/mol |
| Synonyms | N/A |
| Classification / Family | COFs, Porous materials, Aldehyde ligands, Pinacolborane ligands, Conducting polymers |
Chemical Structure
Product Details
| Purity | 98% |
| Melting Point | N/A |
| Appearance | Orange to brown powder |
MSDS Documentation
2,5-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)terephthalaldehyde MSDS Sheet
Literature and Reviews
- Effective one-pot synthesis of (E)-poly(vinylarylenes) via trans-borylation/Suzuki coupling protocol, J. Szyling et al., Green Process Synth., 6, 301–310 (2017); DOI: 10.1515/gps-2016-0217.
- Porous organic frameworks: advanced materials in analytical chemistry, S. Zhang et al., Adv. Sci., 5, 1801116 (2018); DOI: 10.1002/advs.201801116.
- Two dimensional semiconducting polymers, X. Wei et al., Mater. Chem. Front., 4, 3472 (2020); DOI: 10.1039/d0qm00309c.
