TPT10 for low-cost, highly efficient organic polymer solar cells
Available to buy online with free shipping on qualifying orders
Specifications | Pricing and Options | MSDS | Literature and Reviews
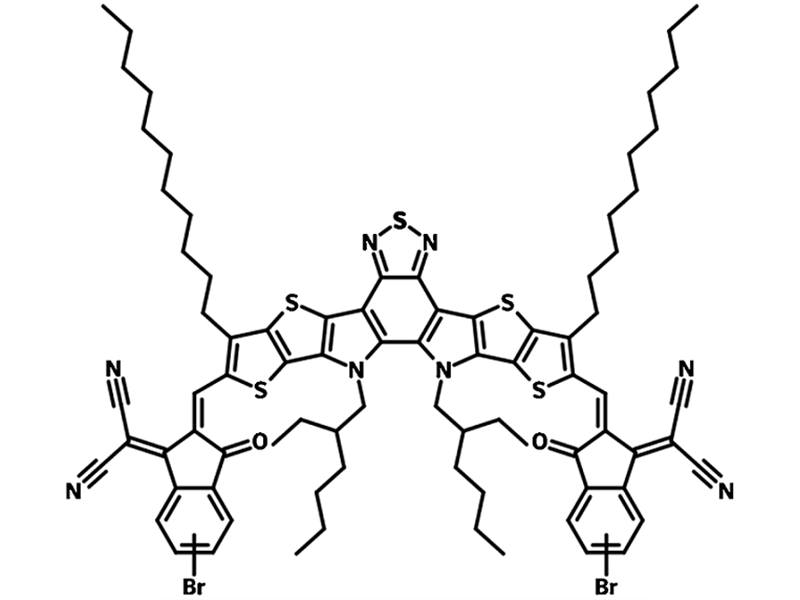
TPT10 (CAS number 2414381-17-0) is a non-fullerene acceptor molecule with a very similar chemical structure to both Y6 and Y7. The only structural difference between TPT10 and Y6 or Y7 is that TPT10 has mono-brominated peripheral end groups. Y6 and Y7, by comparison, have bis-fluorinated and chlorinated end groups respectively. With fewer bromine substitutes, TPT10 shows a better solubility in common solvents. This property is exaggerated in TPT10-C8C12, which has larger butyldodecyl side chains than TPT10.
TPT10 has been successfully used with PTQ10 and PTQ11 to produce low-cost, highly efficient organic polymer solar cells with a reported power conversion efficiency (PCE) of 16.32%. This brings organic photovoltaic (OPV) cells a step closer to viable commercialization.
Device structure: ITO/ZnO/PTQ11:TPT10 (1:1.2):0.5 % 1-CN/MO3/Ag
| Thickness (nm) | VOC (V) | JSC (mA cm-2) | FF (%) | PCE (%) |
| 110 | 0.88 | 24.79 | 74.8 | 16.32 |
We supply TPT10 worldwide. Quantities from 100 mg to 1 g are available to buy online (please contact us for larger orders). In addition, we also stock BTP-4F (Y6), BTP-4Cl (Y7) and TPT10-C8C12. Eligible orders ship free.
General Information
| CAS Number | 2414381-17-0 |
| Chemical Formula | C82H88Br2N8O2S5 |
| Full Name | 2,2'- [[12,13-Bis(2-ethylhexyl)-12,13-dihydro-3,9-dinonylbisthieno[2'',3'':4',5']thieno[2',3':4,5]pyrrolo[3,2-e:2',3'-g][2,1,3]benzothiadiazole-2,10-diyl]bis[methylidyne(5 or 6-bromo-3-oxo-1H-indene-2,1(3H)-diylidene) ]]bis[propanedinitrile] |
| Molecular Weight | 1537.8 g/mol |
| HOMO / LUMO | HOMO = -5.52 eV, LUMO = -3.99 eV [1] |
| Solubility | Chloroform, chlorobenzene and dichlorobenzene |
| Form | Dark blue powder/crystal |
| Synonyms | BTP-2Br, BTIC-2Br-m |
| Classification / Family | BTP series NFAs, n-type non-fullerene electron acceptors, organic semiconducting materials, low band-gap small molecule, small molecular acceptor, organic photovoltaics, polymer solar cells, NF-PSCs |
Chemical Structure
MSDS Documentation
Pricing
| Batch | Quantity | Price |
| M2250A1 | 100 mg | £360 |
| M2250A1 | 250 mg | £720 |
| M2250A1 | 500 mg | £1250 |
| M2250A1 | 1 g | £2300 |
| M2250A1 | 5 g / 10 g | Please contact us for details |
Quantities over 5 g are available with a lead time of 4 - 6 weeks
Literature and Reviews
- High Efficiency Polymer Solar Cells with Efficient Hole Transfer at Zero Highest Occupied Molecular Orbital Offset between Methylated Polymer Donor and Brominated Acceptor, C. Sun et al., J. Am. Chem. Soc., 142, 1465−1474 (2020); DOI: 10.1021/jacs.9b09939.
- Volatilizable and cost-effective quinone-based solid additives for improving photovoltaic performance and morphological stability in non-fullerene polymer solar cells, Y. Zhang et al., J. Mater. Chem. A, 8, 13049-13058 (2020); DOI: 10.1039/D0TA04941G.
- Bromination: An Alternative Strategy for Non‐Fullerene Small Molecule Acceptors, H. Wang et al., Adv. Sci (Weinh), 7(9), 1903784 (2020); DOI: 10.1002/advs.201903784.
Related Products
Semiconducting polymers for bulk heterojunction, OPV, OLED, OFET and perovskite interfaces and solar cell research.


