Y6 (BTP-4F) non-fullerene acceptor molecule for OPVs
Featured in Advance Science, Ossila Y6 solution processed thin-film transistors gained electron mobilities up to 2.4 cm2 V−1 s−1
Specifications | MSDS | Y6 Derivatives | Literature and Reviews
Y6 (CAS number 2304444-49-1) is a popular non-fullerene acceptor (NFA) molecule. The discovery of ITIC and the subsequent boom in the use of NFAs in organic photovoltaic solar cells (OPVs) has led to rapid improvements in device power conversion efficiencies (PCEs). The development of new NFA molecules like Y6 has continued this exciting trend. Using Y6 within a inert environment (such as a glove box), considerable jumps in solar cell performance have been achieved.
Highly Efficient NFA
With highly conjugated core
Wide Optical Absorption
Induced by intermolecular interactions
Worldwide Shipping
Quick and reliable shipping
High Purity
>98% high purity
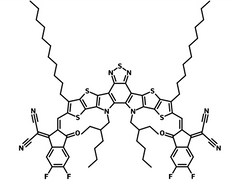
Also known BTP-4F, Y6 is a highly conjugated electron deficient organic semiconductor with an A-DAD-A structure. The Y6 molecule is composed of a fused thienothienopyrrolo-thienothienoindole (TTP-TTI) core base and 2-(5,6-difluoro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (2FIC) end units. These 2FIC end units are believed to promote intermolecular interactions and enhance optical absorption. The absorption spectrum of Y6 has a maximum at around 810 nm and extends to 1100 nm. This means that Y6 and its polymer blends have the potential to absorb light across the entire visible and near infra-red spectrum.
The benzothiadiazole core of Y6 allows for the creation of solar cells using the polymer PBDB-T-2F (PM6) as an electron donor. Impressive power conversion efficiencies of up to 15.7% have been demonstrated in optimized single-junction solar cells with both conventional and inverted architectures using Y6 and PM6. Device structure: ITO/PEDOT:PSS/PM6:Y6/PDINO/Al.
Y6 (BTP-4F) from Ossila was used in the high-impact paper (IF 29.37), Triplet-Charge Annihilation in a Small Molecule Donor: Acceptor Blend as a Major Loss Mechanism in Organic Photovoltaics, J. Marin-Beloqui et al., Adv. Energy Mater., 2100539 (2021); DOI: 10.1002/aenm.202100539. Y6 Organic Thin-Film Transistors with Electron Mobilities of 2.4 cm2 V−1 s−1 via Microstructural Tuning, E. Gutierrez-Fernandez et al., Adv. Sci., 9 (1), 2104977 (2022); DOI: 10.1002/advs.202104977.
We supply high purity Y6 to institutions around the world. Quantities from 50 mg to 5 g are available to buy online (please contact us for larger orders) and eligible orders ship free.
General Information
| CAS Number | 2304444-49-1 |
| Chemical Formula | C82H86F4N8O2S5 |
| Purity | >98% (1H NMR) |
| Full Name | 2,2'-((2Z,2'Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2",3’':4’,5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile |
| Molecular Weight | 1451.93 g/mol |
| HOMO / LUMO | HOMO = -5.65 eV, LUMO = -4.10 eV [1] |
| Synonyms | TTPTTI-4F, BTPTT-4F, BTP-4F, Y6F, BTP-4F-8 |
| Classification / Family | NFAs, n-type non-fullerene electron acceptors, organic semiconducting materials, low band-gap small molecule, small molecular acceptor, organic photovoltaics, polymer solar cells, NF-PSCs |
Chemical Structure
MSDS Documentation
Y6 Derivatives
The success of the electron-deficient-core-based fused ring design of Y6 has led to the development of other non-fullerene acceptors for solar cells based on the same principle. The different alkyl substituents on the derivatives influence their solubility, charge transport properties and the morphology of the resulting bulk heterojunction solar cell.
Literature and Reviews
- Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core, J. Yuan et al., Joule (2019); doi: 10.1016/j.joule.2019.01.004.
- Fluorination vs. chlorination: a case study on high performance organic photovoltaic materials, Y. Zhang et al., Sci. China. Chem., 61 (10), 1328-1337 (2018); doi:10.1007/s11426-018-9260-2.
- Achieving over 16% efficiency for single-junction organic solar cells, B. Fan et al., Sci. China Chem., 62, 6 746-752 (2019); doi: 10.1007/s11426-019-9457-5.
Related Products
Semiconducting polymers for bulk heterojunction, OPV, OLED, OFET and perovskite interfaces and solar cell research.






