Graphene Batteries: How Is Graphene Used In Batteries

Graphene batteries are advanced energy storage devices. Graphene materials are two-dimensional and are typically made solely of carbon. They can also be incorporated into existing systems such as lithium-ion (Li-ion) or aluminum-ion (Al-ion) batteries. Graphene's high conductivity, large surface area, and flexibility enhance battery performance, with the most popular incorporation being at the electrodes. Traditional Li-Ion batteries are known to be toxic, unsustainable and flammable but graphene can help alleviate these problems.
On This Page
Why Use Graphene In Batteries?
The first Li-ion battery was developed in 1976, a similar time to Al-ion batteries. However, graphene was only discovered in 2004. The use of graphene in batteries is much more recent, but despite this they can still outperform Li-ion batteries in several areas.
Typically, Li-ion batteries charge within a couple of hours. Graphene enhanced batteries offer much faster charging, recent reports suggest a full charge in less than half an hour. The capability to charge faster also helps to extend the battery's lifetime. Graphene batteries are reported to last about 5 times longer than Li-ion batteries.
One of the most important benefits of incorporating graphene into batteries is the improved safety. Li-ion batteries are becoming infamous for causing fires, however graphene's stability and heat dissipation make it a non-flammable option. Handling graphene is also much safer than lithium as it is non-toxic.
Additionally, graphene is often described as a more sustainable alternative to Li-ion or Al-ion batteries. Unlike lithium, aluminum, cobalt, and nickel, which are mined from finite natural sources, graphene is a lab-made material, offering a more sustainable approach to battery production.
How Graphene Is Used In Batteries
Batteries release and store energy by converting between chemical potential energy and electrical energy. This conversion is a result of reduction and oxidation (redox) reactions. Batteries consist of:
- two electrodes: cathode and anode
- electrolyte
- separator
- power supply
A voltage drives ions out of the cathode, through the electrolyte and to the anode where redox reactions take place. The electrons released create an electrical current which is then transferred to an external circuit. The direction of charge movement between the cathode and anode are determined by whether the battery is charging (restoring stored energy) or discharging (releasing stored energy).
Electrodes
At the electrodes, ions either gain electrons (reduction) or lose electrons (oxidation), these redox reactions are what make batteries work. An electrode’s performance is based on how well that material can transfer charge.
Graphene has a high surface area and capacity which helps to store more ions within its layers. This gives graphene batteries a higher capacity and longer lifetime. Therefore, graphene is an appropriate material for both cathode and anode applications.
Electrodes are one of the most influential parts of the battery. Therefore research into graphene cathodes and anodes is very popular.
Hybrid Graphene Lithium-Ion Batteries
Graphene is not widely used as a direct electrode material in lithium-ion batteries (LIBs) due to poor coulombic efficiency, high charge–discharge platform, and cycle instability. However, its excellent conductivity makes it valuable as a support, mediator, framework, or electrode modifier in composite electrodes.
Graphene in Cathodes
Graphene improves cathode performance by enhancing electron/ion transport and diffusion.
- LiMn2O4/rGO composites: Microwave-assisted hydrothermal synthesis disperses nano-sized LiMn2O4 on rGO, yielding high capacity, rate capability, and stable cycling.
- LiFePO4 with graphene sheets: Dropwise addition of graphene in DMF improves capacity (187–208 mAh g-1) by preventing agglomeration and volume expansion.
- Other methods: Self-assembly, wet chemistry, mechanical mixing, micro-emulsion, ball-milling, solvothermal synthesis, and spray drying.
Graphene in Anodes
Graphene’s high surface area (2620 m2 g-1), thinness, and flexibility shorten ion diffusion distances, improve conductivity, and buffer structural stress.
- Sn–graphene composites: Hydrothermal synthesis with carbon coating accommodates Sn volume changes, improving cycling and conductivity.
- Transition metal oxides: Graphene prevents agglomeration and enhances conductivity in oxide-based anodes.
- SiOx/TiO2@MLG composites: TiO2 exfoliates graphite into multilayer graphene, which wraps SiOx particles. This design boosts conductivity, buffers volume changes, and achieves 1484 mAh g-1 capacity, maintaining stability over 1200 cycles.
History of Graphene Batteries
Graphene was discovered in 2004, and single layers were only accessed in 2010. After this, the benefits of graphene enhanced batteries were recognized almost immediately which has lead to rapid advancements in this area.
- 2004 – Discovery of graphene.
- October, 2010 – Nobel prize for accessing monolayers of graphene
- June, 2010 – 30-inch graphene sheet fabricated
- February, 2011- Graphene based bendable battery is made
- October, 2011 - UK invest £50 million into commercial graphene opportunities
- December, 2011 – First commercial graphene device
- January, 2013 – Graphene based Li-ion batteries come to market
- June, 2014 – Commercial graphene enhanced battery product
- June, 2015 – Samsung use graphene to double the capacity of a Li-ion battery.
- November, 2016 – Huawei use graphene to enhance battery
- December, 2022 – NASA begin to test graphene battery for space applications
- June, 2023 – Commercialization of graphene enhanced Li-S batteries
Benefits of Graphene Batteries
It's clear from the rapid development, that the benefits of incorporating graphene into batteries are significant. Beneficial properties include a large surface area, high energy storage, flexibility and heat management.
Large surface area
A higher surface area results in more active sites at the electrodes and so improved charge storage. This subsequently leads to faster charging rates as well as higher strength.Graphene has a huge surface area of 2630 m2 per gram. That’s enough to cover about 10 tennis courts in just 1 gram. Compared to lithium where 1 gram can only cover 1 table tennis court.
High energy storage capability
Currently, commercial Li-ion batteries have energy densities less than 250 Wh kg-1. Whereas those which incorporate graphene have reached around 1000 Wh kg-1. Therefore graphene batteries can hold up to 4 times more charge than Li-ion batteries. For example, fluorine doped graphene has found to have an energy density as high as 1073 Wh kg-1. This increase in energy density allows for smaller and therefore more lightweight batteries with longer lifetimes.
Flexibility
Graphene’s 2D structure means it can easily deform and bend, making it highly flexible. This makes it easier to incorporate into appliances and even allows for rollable devices.
Fast charging & longer lifetimes
The layered structure of graphene means more active sites and so quicker and more efficient charging. Research on graphene aluminum-ion batteries has shown charging times are up to 70 times faster than Li-ion batteries. Faster charging also enables longer cell lifetimes, graphene enhanced batteries can have a lifetime 3 times longer than that of a Li-ion battery.
Non-flammable
A major drawback of Li-ion batteries is their potential to overheat and catch fire. Graphene, however, is non-flammable and so a much safer alternative. Graphene has even been used to lower the operating temperatures of some batteries. Decreases of up to 25°C have been seen in graphene enhanced Li-ion batteries.
Applications of Graphene Batteries
The global graphene market is expected to increase by a rate of 35 to 40% each year. With continuing research and advancements, graphene batteries are capable of a wide range of applications.
Consumer Devices
Many consumer devices, such as phones and laptops, use lithium-ion batteries. In 2023 in the UK, fire services were called to 921 lithium-ion battery fires, a 46% increase from 2022. By exploiting the thermal stability of graphene these fires can be prevented.
Space Applications
The benefits of graphene have also been recognized for space applications. Their high energy storage, long lifetimes and good temperature performance has put them under investigation for satellite applications.
Electric vehicles (EVs)
EVs are becoming increasingly popular as a sustainable alternative. However, the batteries currently being used require extensive cooling systems to prevent them overheating. Graphene’s excellent thermal management means it would not require such cooling systems. Therefore, graphene battery systems would be more compact than the current Li-ion options.
Power Tools
Cordless power tools are not only more convenient but safer too. With the faster charging times of graphene batteries, fewer replacements are needed making them a practical alternative for remote construction sites.
Battery-Supercapacitor Hybrids
A supercapacitor is used when energy is needed in short, sharp bursts. By combining the quick energy supply of supercapacitors and the high storage of batteries, the disadvantages of both can be overcome in a battery-supercapacitor hybrid (BSH). Li4Ti5O12 is often used as an electrode in capacitors. When coupled with N-doped graphene, a BSH can be formed and this has achieved storage of up to 70 Wh kg-1.
What Is Graphene?
Graphene consists only of carbon and, as a 2D material, exists in thin sheets. The carbons of graphene each bond to three others in the x-y plane with nothing in the z plane. Therefore, a single layer (monolayer) of graphene is only one atom thick. The carbon atoms are arranged in benzene rings and appear as a honeycomb-like structure.

Accessing these monolayers of graphene was discovered by Novoselov and Geim, who were awarded a Nobel Prize in 2010. Single layers of graphene were accessed using the same scotch tape you may use to wrap presents. Since then, graphene has been increasingly investigated. Graphene’s high strength, high thermal conductivity, and ability to transport charges makes it an obvious choice for electrical storage devices such as capacitors and batteries.
Variations of graphene used in batteries
Besides graphene, other carbon-based materials like holey graphene, graphene oxide, graphene nanotubes, and doped graphene have also shown potential in enhancing battery properties.

Holey Graphene
Holes in the graphene layers mean faster ion transportation. Holey graphene can be incorporated in the anode or cathode. Therefore, holey graphene can lead to faster charging times and greater storage.

Graphene Oxide (GO)
GO can be easily dispersed in solution and processed at high concentrations. The oxygen functional groups of GO help with ion absorption and so can increase batteries capacity. These factors are beneficial to electrodes, GO has been implemented in cathodes, for example.
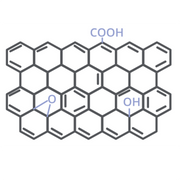
Reduced Graphene Oxide (rGO)
By chemically reducing GO, there are fewer defects. rGO can improve battery stability as well as conductivity, as fewer functional groups obstruct the path of charges. Research shows rGO in cathode applications.
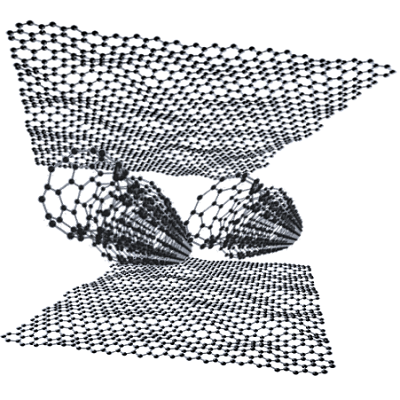
Graphene Carbon Nanotubes
Graphene nanotubes can act as regulators in lithium batteries. They can help with storage behaviors and reduce defects forming.
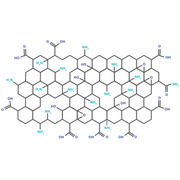
N-Doped Graphene Oxide
Doping with nitrogen can repair defects in GO. N-doped GO has been used successfully in anodes.
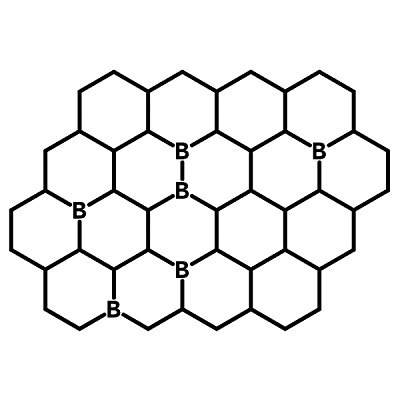
B-Doped Graphene Oxide
Doping with boron increases the materials capacity and shows good stability. B-doped GO has been used as an anode material in potassium-ion batteries.
Graphene Materials
Future Outlook for Graphene Batteries
Reduce fabrication costs
Graphene sheets can be grown in a variety of ways but most commonly by chemical vapor deposition (CVD). CVD involves depositing materials, in their gas state, onto a heated solid. These methods, however, are energy intensive and therefore expensive. The performance of graphene batteries relies on high purity and low defect graphene, achieving these also add to the cost of manufacturing. These lab-based methods have proved effective for small-scale devices, however cost will become more important as the industry grows larger.
Improve charge capacity
Graphene has a theoretical capacity between 100 and 1000 mAh g-1, depending on how it was made and any defects present. Capacities as high as 1264 mAh g-1 have been achieved using a graphene anode in a Li-ion battery. However Li-ion batteries alone have reached capacities of 3860 mAh g-1. Despite graphene's capacity being only a third of what lithium can achieve, it is a relatively new material, and research is still ongoing.
Minimize environmental Impact
Although graphene is not a mined material, like lithium or aluminum, it is also not a fully sustainable alternative. Methods of fabricating graphene are energy intensive and it is difficult to recycle. There is potential to use waste carbon products to produce graphene, however this may introduce a significant number of defects.
Learn More
 Graphene Battery vs Lithium-Ion Battery
Graphene Battery vs Lithium-Ion Battery
This page discusses the pros and cons of Lithium-ion (Li-ion) batteries and graphene batteries and the future outlook for battery research.
Learn more...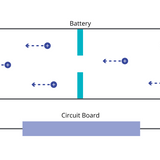 Lithium-Ion Battery Components and Working Principle
Lithium-Ion Battery Components and Working Principle
Lithium-ion batteries operate based on electrochemical reactions, specifically redox reactions involving lithium and sometimes other redox-active elements. These reactions result in the movement of lithium ions between the electrodes and the flow of electrons through an external circuit.
Read more...
References
- Two years and a pandemic later, fast-charging graphene..., Sage, Digital Trends (2021)
- Electric Field Effect in Atomically Thin Carbon Films, Novoselov et al, Science (2004)
- Nobel Prize 2010: Andre Geim & Konstantin Novoselov, Gerstner, Nature Physics (2010)
- Roll-to-roll production of 30-inch graphene films for transparent..., Bae et al, Nature Nanotechnology (2010)
- Flexible energy storage devices based on graphene paper, Gwon, Energy & Environmental Science (2011)