Bathocuproine (BCP)
CAS Number 4733-39-5
Charge Transport Layer Materials, Electron Injection Layer Materials, Electron Transport Layer Materials, High Purity Sublimed Materials, Materials, OLED Materials,Bathocuproine, one of the most popular HBL materials used in organic electronics
Aids the improvement of PCE of polymer organic solar cells when used as a buffer layer, Sublimed ≥99%, CAS No. 4733-39-5
Specifications | MSDS | Literature and Reviews
Bathocuproine (BCP), CAS number 4733-39-5, is a wide-band-gap material and has a high electron affinity. When it is embedded into organic electronic devices, bathocuproine acts as an electron-transport layer (ETL) and exciton-blocking barrier (EBL) which prohibits exciton diffusion process towards the Al electrode. It has a phenanthroline core with methyl and phenyl substituents. The electron-donating nitrogen atoms are able to complex with metal electrodes, improving charge transport properties and stability.
Bathocuproine is one of the most commonly used buffer layer between acceptor and cathode layers. The introduction of the buffer layer can greatly improve the PCE of polymer organic solar cells. Bathocuproine is one of the most popular hole-blocking layer materials that is used in organic electronics, including perovskite solar cells.
Bathocuproine (BCP) from Ossila was used in the high-impact paper (IF 30.85), Engineering Band-Type Alignment in CsPbBr3 Perovskite-Based Artificial Multiple Quantum Wells, K. Lee et al., Adv. Mater., 33 (17), 2005166 (2021); DOI: 10.1002/adma.202005166; and paper (IF 22.0), Suppressing PEDOT:PSS Doping-Induced Interfacial Recombination Loss in Perovskite Solar Cells, Y. Chin et al., ACS Energy Lett., 7 (2), 560–568 (2022); DOI: 10.1021/acsenergylett.1c02577.
It was demonstrated that a bathocuproine buffer layer reduces nonradiative recombination of excitons at the C60 –Al interface. Its most important function is to establish an Ohmic contact between the C60 film and the Al electrode in photovoltaic devices [4].
General Information
| Full name | 2,9-Dimethyl-4,7-diphenyl-1,10-phenanthroline |
|---|---|
| Synonyms |
|
| CAS number | 4733-39-5 |
| Molecular formula | C26H20N2 |
| Molecular weight | 360.45 g/mol |
| HOMO / LUMO | HOMO ~ 6.4 eV LUMO ~2.9 eV |
| Classification / Family | Electron-transport layer (ETL), Electron-injection layer (EIL), Hole-blocking layer, OFET, OLED, Organic Photovoltaics, Perovskite solar cells, Sublimed materials. |
Product Details
| Purity | >99.5% (sublimed) >98.0% (unsublimed) |
|---|---|
| Melting point | 280-282°C (lit.) |
| Appearance | Light yellow powder |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials.
Chemical Structure

Device Structure(s)
| Device structure | ITO/DNTPD* (60 nm)/NPB (20 nm)/mCP (10 nm)/mCP:FIrpic (25 nm)/CBP:Ir(piq)2acac (5 nm)/BCP (5 nm)/Alq3 (20 nm)/LiF (1 nm)/Al (200 nm) [5] |
|---|---|
| Color | White |
| EQE@500 cd/m2 | 8.2 % |
| Current Efficiency (@500 cd/m2) | 12.7 lm W−1 |
| Device structure | ITO/NPB (30 nm)/NPB: DCJTB: C545T* (10 nm)/NPB (4 nm)/DNA (8 nm)/BCP (9 nm)/Alq3 (30 nm)/LiF (1 nm)/Al (100 nm) [6] |
|---|---|
| Color | White |
| Max. Luminance | 13,600 cd/m2 |
| Max. Current Efficiency | 12.3 cd/A |
| Max. Power Efficiency | 4.4 lm W−1 |
| Device structure | ITO/2T-NATA (17 nm)/TPAHQZn* (25 nm)/NPBX* (15 nm)/BCP (8 nm)/ Alq3 (35 nm)/LiF (0.5 nm)/Al (120 nm) [7] |
|---|---|
| Color | White |
| Max. EQE | 17.5% |
| Max. Luminance | 12,930 cd/m2 (12 V) |
| Max. Current Efficiency | 2.66 cd/A (10 V) |
| Device structure | ITO/ NPB(60 nm)/Alq3:DCM(7nm)/BCP(12 nm)/ Alq3(36nm)/ MgAg(200 nm) [8] |
|---|---|
| Color | Red |
| Max. Luminance | 1, 000 cd/m2 |
| Max. Current Efficiency | 5.66 cd/A |
| Device structure | ITO/α-NPD* (50 nm)/7%-Ir(ppy)3:CBP (20 nm)/BCP (10 nm)/Alq3 (40 nm)/Mg–Ag (100 nm)/Ag (20 nm) [9] |
|---|---|
| Color | Green |
| Max EQE | (12.0±0.6)% |
| Max. Powder Efficiency | (45±2) lm W−1 |
*For chemical structure information please refer to the cited references.
Characterization
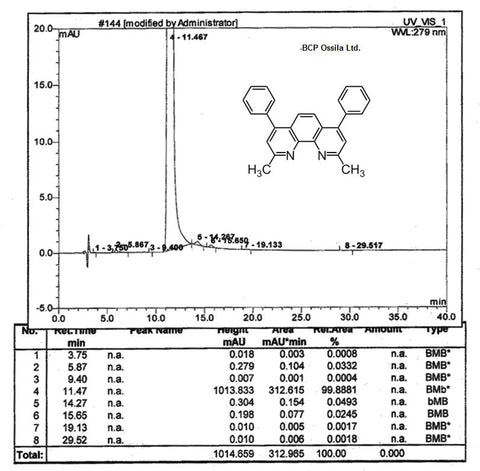
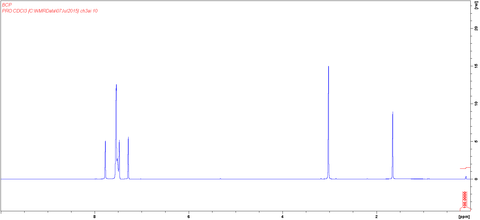
MSDS Documentation
Literature and Reviews
- Detailed analysis of bathocuproine layer for organic solar cells based on copper phthalocyanine and C60, J. Huang et al., J. Appl. Phys., 105, 073105 (2009)
- On the Role of Bathocuproine in Organic Photovoltaic Cells, H. Gommans et al., Adv. Funct. Mater., 18, 3686-3691 (2008)
- A Blue Organic Light Emitting Diode, Y. Kijima et al., J. Appl. Phys., 38, 5274-5277 (1999)
