Ir(ppy)3
CAS Number 94928-86-6
Dopant Materials, Green Dopant Materials, High Purity Sublimed Materials, Materials,Ir(ppy)3, one of the most successful green-triplet emitters for OLED devices
High quantum yields and thermal stability, Tris(2-phenylpyridine)iridium(III), CAS No. 94928-86-6, Sublimed ≥99.0%
Tris(2-phenylpyridine)iridium(III), Ir(ppy)3, is used widely in organic light-emitting diodes (OLEDs) due to its high quantum yields and thermal stability.
Utilizing all of its singlet and triplet excitons for the emission, this green light emitting Ir(ppy)3 exhibits a very bright phosphorescence with an internal quantum yield of almost 100% [1, 2, 3]. It is one of the most successful green-triplet emitters in the rapidly developing field of OLED display technology.
General Information
| CAS number | 94928-86-6 |
|---|---|
| Chemical formula | C33H24IrN3 |
| Molecular weight | 654.78 g/mol |
| Absorption | λmax 282, 377 nm in THF |
| Fluorescence | λem 513 nm in THF |
| HOMO/LUMO | HOMO 5.6 eV, LUMO 3.0 eV |
| Synonyms | Tris[2-phenylpyridine]iridium(III), Tris(2-phenylpyridinato)iridium(III), Tris[2-phenylpyridine-c2,n]iridium(III), Irppy3 |
| Classification / Family | Organometallic complex, Green emitter, Phosphorescence dopant OLEDs, Sublimed materials |
Product Details
| Purity |
>99.5% (sublimed) >98.0% (unsublimed) |
|---|---|
| Melting point | 451 °C |
| Color | Yellow powder |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials.
Chemical Structure
Device Structure(s)
| Device structure | ITO/α-NPD* (50 nm)/7%-Ir(ppy)3:CBP (20 nm)/BCP (10 nm)/tris(8-hydroxyquinoline)aluminum (Alq3) (40 nm)/Mg–Ag (100 nm)/Ag (20 nm) [4] |
|---|---|
| Color | Green |
| Max EQE | (12.0±0.6)% |
| Max. Powder Efficiency | (45±2) lm W−1 |
| Device structure | ITO/α-NPD* (40 nm)/6%-Ir(ppy)3:CBP (20 nm)/BCP (10 nm)/tris(8-hydroxyquinoline)aluminum (Alq3) (20 nm)/Mg–Ag (100 nm)/Ag (20 nm) [5] |
|---|---|
| Color | Green |
| Max EQE | 8% |
| Max. Current Efficiency | 28 cd/A |
| Max. Powder Efficiency | 31 lm W−1 |
| Device structure | ITO/PEDOT:PSS (50 nm)/poly-TCZ (35 nm)/1*:Ir(ppy)3 (94:6 wt%)(20 nm)/TAZ (50 nm)/LiF (2.5 nm)/Al (40 nm)/Ag (100 nm) [6] |
|---|---|
| Color | Blue |
| Max. Luminance | 47,000 cd/m2 |
| Max. Current Efficiency | 81.1 cd/A |
| Max. EQE | 25.2% |
| Max. Power Efficiency | 46.8 lm W−1 |
| Device structure | ITO/MoO3 (3 nm)/CBP: 20 wt% Ir(ppy)3: 4 wt% FIrpic (30 nm)/TAZ (50 nm) [7] |
|---|---|
| Color | Green |
| Max. Luminance | 27,524 cd/m2 |
| Max. Current Efficiency | 71.2 cd/A |
| Device structure |
ITO/NPD/5%BCzVBi:CBP/CBP/4%PQIr*:CBP/5%Ir(ppy)3:CBP/CBP/5%BCzVBi:CBP/ LiF/Al [8] |
|---|---|
| Color | White |
| Max EQE | 11.0 ± 0.3% |
| Max. Power Efficiency | 22.1 ± 0.3 lm W−1 |
| Device structure | ITO/0.4 wt% F4TCNQ doped α NPD (30 nm)/ 5 wt% Ir (ppy)3 doped CBP (50 nm)/BPhen (30 nm)/20 wt% TCNQ mixed BPhen (1.5 nm)/Al [9] |
|---|---|
| Color | Green |
| Luminance@15 V | 1,320 cd/m2 |
| Power Efficiency@14 V | 56.6 lm W−1 |
| Current Efficiency@14 V | 23.17 cd/A |
*For chemical structure information please refer to the cited references
Characterizations
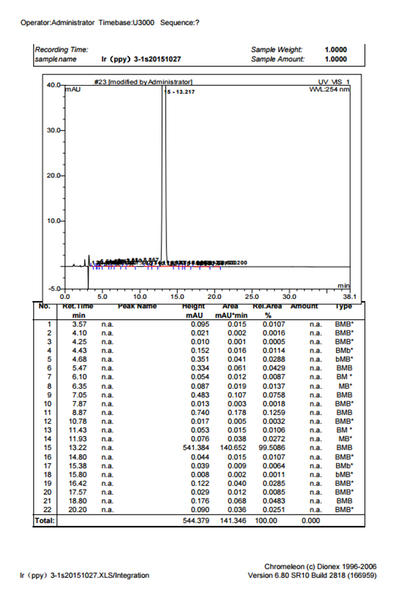
Pricing
| Grade | Order Code | Quantity | Price |
|---|---|---|---|
| Sublimed (>99.5% purity) | M481 | 100 mg | £250 |
| Unsublimed (>98.0% purity) | M482 | 250 mg | £290 |
| Sublimed (>99.5% purity) | M481 | 250 mg | £500 |
| Unsublimed (>98.0% purity) | M482 | 500 mg | £580 |
| Sublimed (>99.5% purity) | M481 | 500 mg | £850 |
| Unsublimed (>98.0% purity) | M482 | 1 g | £980 |
| Sublimed (>99.5% purity) | M481 | 1 g | £1450 |
MSDS Documentation
Literature and Reviews
- Absorption and emission spectroscopic characterization of Ir(ppy)3, W. Holzer et al., Chem. Phys., 308(1-2), 93-102 (2005), doi:10.1016/j.chemphys.2004.07.051.
- The Triplet State of fac-Ir(ppy)3, T. Hofbeck et al., Inorg. Chem., 49 (20), 9290–9299 (2010), DOI: 10.1021/ic100872w.
- High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer, M. A. Baldo et al., Nature 403, 750-753 (2000) | doi:10.1038/35001541.

