PEDOT Polymer Blends

PEDOT:PSS (or Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate, CAS 155090-83-8) is a transparent conductive polymer. Due to its unique combination of conductivity, transparency, ductility, and ease of processing, PEDOT:PSS has become a benchmark material in thin-film electronic fabrication.
The properties of PEDOT:PSS vary between dispersions, hence its versatility. Since PEDOT is conductive and PSS is insulating, the conductivity of the resulting polymer depends on the ratio between the two ionomers and the microstructure of the film. Similarly, a higher presence of PSS at the surface will result in an increased work function.
Based in different solvents and offering different conductivity, high quality PEDOT products at Ossila give a great application spectrum ranging from transparent electrodes, OLEDs, OPVs to sensors, touch screen, conductive textile and smart wearable electronics.
Our PEDOT products are supplied in light resistant bottles with temperature indicators.
Jump to: Browse PEDOT materials | Choose the right PEDOT | Frequently asked questions | Learn more
Browse PEDOT Materials
Filter by type:
Filter by solvent:
Filter by sheet resistance:
Filter by deposition method:
Filter by applications:
PEDOT:PSS from Ossila was featured in the high-impact paper (IF 30.85), A Wearable Supercapacitor Based on Conductive PEDOT:PSS-Coated Cloth and a Sweat Electrolyte, L. Manjakkal et al., Adv. Mater., 1907254 (2020); DOI: 10.1002/adma.201907254.
Choose the Right PEDOT Material

Choosing the right PEDOT product for you could be a difficult task. Each has a different base solvent, conductivity, viscosity, and even composition. At Ossila, we have a range of PEDOT:PSS and other PEDOT polymer blends. They are available in different solvents for applications in OLED, OPV, and sensors, and are suitable for different deposition methods, i.e. spin coating, inkjet printing and screen printing.
PEDOT Products by Sheet Resistance
| <100 Ω/sq | 100-1000 / Ω/sq | 1000 - 10E4 / Ω/sq | 10E4 - 10E6 / Ω/sq | +10E6 / Ω/sq |
|---|---|---|---|---|
|
HY E (AgNW) |
PH 1000 F HC Solar F 020 F ET S V3 S V3 STAB S V4 S V4 STAB P T4 F 100T P JET X N |
P T4 |
P T4 HTL Solar |
HTL Solar 3 HIL 8 Al 4083 P JET (OLED) P T4 HTL Solar |
Back to: PEDOT products
PEDOT Products by Application
| OLED | OPV | Transistors | Sensor | Transparent Electrodes | Conductive Textiles | LC Writing Boards |
|---|---|---|---|---|---|---|
|
Al 4083 PH 1000 HIL 8 P JET (OLED) HY E (AgNW) P JET X N |
Al 4083 PH 1000 HTL Solar HTL Solar 3 F HC Solar HTL Solar 4 HY E (AgNW) |
PH 1000 S V3 S V3 STAB S V4 S V4 STAB P JET (OLED) |
HTL Solar F HC Solar S V3 S V3 STAB S V4 S V4 STAB F ET P T4 HY E (AgNW) |
PH 1000 S V3 S V3 STAB S V4 S V4 STAB F 020 F ET P T4 HY E (AgNW) |
F 020 F ET S V3 S V3 STAB S V4 S V4 STAB PH 1000 |
F 020 F ET P T4 PH 1000 HY E (AgNW) F 100T |
Back to: PEDOT products
PEDOT Products by Solvent Base
| Water | Toluene | Anisole | Butyl benzoate | Glycols (Paste) | |
|---|---|---|---|---|---|
|
Al 4083 PH 1000 HTL Solar F HC Solar HY E (AgNW) P JET X N |
P JET (OLED) F 020 F ET P T4 F 100T |
HTL Solar 3 |
HTL Solar 4 |
HIL 8 |
S V3 S V3 STAB S V4 S V4 STAB |
Back to: PEDOT products
PEDOT Products by Deposition Method
| Coating* | Inkjet Printing | Screen Printing | |
|---|---|---|---|
|
Al 4083 PH 1000 HTL Solar HTL Solar 3 HTL Solar 4 F HC Solar F 100T |
HIL 8 F 020 F ET S V3 S V3 STAB S V4 S V4 STAB HY E (AgNW) P T4 |
P JET (OLED) P JET X N |
S V3 S V3 STAB S V4 S V4 STAB |
* Spin coating, spray coating, slot die coating, doctor-blade coating, dip coating
Back to: PEDOT products
Frequently Asked Questions
How Should PEDOT Materials be Stored?
The recommended storage temperature of PEDOT polymer blends is between 5 and 10 °C. It is recommended that you store the dispersion at the front of a refrigerator. The product is not usable if frozen, so should be kept away from the back of the refrigerator.
The dispersions can tolerate up to a week outside of the refrigerator (e.g. during shipping) without negative consequences for the PEDOT polymer performance. Over time at elevated temperatures, the PEDOT material can phase separate, aggregate, and form a solid that drops to the bottom of the bottle, reducing performance. Brief heating to a maximum of 50 °C has no adverse effect on product properties.
What is the Shelf Life of PEDOT Materials?
When stored at 5 to 10 °C, PEDOT materials will give you consistent performance over 12 months if used regularly. The official recommended shelf life from the manufacturer is 9 months after production if the dispersion is never used or disturbed. After this time, the dispersion will gradually separate or sediment at a very slow rate, resulting in lower concentrations and thinner films.
Material degradation is mitigated by frequent use of the dispersion due to the gentle agitation when decanting. Through constant use, it is possible to use a single bottle of a PEDOT polymer blend for many years without harming device performance.
The shelf life of other PEDOT polymer blends is the same.
Learn More
PEDOT synthesis involves the oxidative chemical or electrochemical polymerization of EDOT monomer.
Read more...PEDOT:PSS has conductivities in the range of 10-4 - 103 S cm-1. PEDOT:PSS is conductive because it contains the conjugated intrinsically conductive polymer (ICP) PEDOT.
Read more...PEDOT:PSS work function ranges 4.8 - 5.2 eV for commercially available products.
Read more...PEDOT:PSS has a unique combination of electrical, optical, mechanical, and thermoelectric properties. It is one of the most widely used intrinsically conducting polymer (ICP) blends. As a result of it’s desirable properties it has received significant attention in the field of organic optoelectronics, particularly as a p-type semiconductor. This versatile material exhibits a range properties that make it suitable for various applications.
Read more...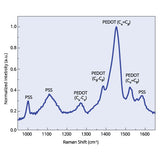 PEDOT:PSS Characterization using Raman Spectroscopy
PEDOT:PSS Characterization using Raman Spectroscopy
The characterization of PEDOT:PSS is essential for determining the relationship between the materials structure, morphology and resulting properties. This is particularly important for their application in electronic devices where their electronic and optical properties are crucial in determining device performance. Some of the key characterization techniques include; Raman Spectroscopy, X-ray, Photoelectron Spectroscopy, Fourier Transform Infrared Spectroscopy and X-ray Diffraction
Read more...Deposition
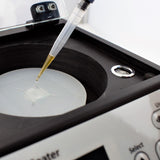 How to Spin Coat PEDOT:PSS
How to Spin Coat PEDOT:PSS
For the deposition of thin films of PEDOT:PSS on a freshly prepared surface, we recommend using a vacuum-free spin coater and following this five-step process:
Read more... PEDOT Coating and Solution Processing
PEDOT Coating and Solution Processing
Methods for depositing PEDOT:PSS, are crucial for fabricating various organic electronic devices. These methods play a significant role in determining the film thickness and morphology, electrical properties, and performance of PEDOT-based materials.
Read more...Applications
 PEDOT:PSS in Solar Cells
PEDOT:PSS in Solar Cells
PEDOT:PSS layers are often used in third generation photovoltaics like organic or perovskite solar cells. It is an attractive material for these applications due to its:
Read more...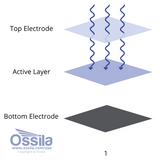 PEDOT:PSS Electrodes
PEDOT:PSS Electrodes
The choice of electrode is very important in many optoelectronic devices. Solar cells, LEDs and photodetectors all need an electrode on either side of the device. These electrodes should meet the following criteria.
Read more... PEDOT:PSS Applications
PEDOT:PSS Applications
PEDOT:PSS stands out as a promising conductive polymer due to its large range of conductivities, transparency, flexibility, and ease of processing.
Read more...Two commonly used materials for hole selective layer (HSL) or hole transport layer (HTL) are PEDOT:PSS (poly(3,4-ethylenedioxythiophene) polystyrene sulfonate) and self-assembled monolayers (SAMs). Each offers distinct advantages and limitations that impact their effectiveness in solar cells.
Read more...Literature and Reviews
Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates, H. J. Snaith et. al. Nature Communications, 4, (2013) DOI: 10.1038/ncomms3761
High efficiency stable inverted perovskite solar cells without current hysteresis, M. Grätzel et. al. Energy Environ. Sci. 8, (2015) 2725-2733 DOI: 10.1039/c5ee00645g

















