Anode Materials

The anode is the electrode where oxidation (loss of electrons) occurs, meaning it releases electrons during battery discharge. Anode active materials are essential for facilitating this process and play a central role in determining a battery’s overall performance, capacity, stability, and safety.
Energy storage is a key area of research for meeting growing global energy demands. A wide range of battery chemistries are being explored, including popular alkali metal ion storage such as lithium and sodium. Each system requires anode materials tailored to specific electrochemical behaviors and performance requirements. Depending on the charge-storage mechanism; intercalation-type, conversion-type or alloy-type battery, there are many suitable anode active materials, each offering unique advantages and challenges.
Anode Active Materials

Carbon-based Anode Materials
Carbon based anode materials are the most widely used, especially in terms of commercial lithium-ion batteries. These materials include graphite, graphene, hard carbon, carbon nanotubes and more. Li+ can be intercalated into and subsequently extracted from these materials without changes to their structure or volume. This is an important characteristic for intercalation-typed anodes to ensure battery stability and safety. They must also have reasonable cycling stability and excellent capacity retention under high-rate charge/discharge process.
Graphite
Graphite powder is the most widely used anode material in terms of intercalation-type batteries because of its high Coulombic efficiency and long cycle performance. The theoretical specific capacity of graphite is 372 mAh/g, which is only about one-tenth of that for Li. A graphite anode stores up to one Li+ for every six carbon atoms between its graphene layers. Therefore, capacity is an area that can be improved when it comes to graphite based anodes.
Carbon Nanotubes (CNTs)
Carbon nanotubes are known for their large aspect ratio, high electrical conductivity, high specific surface area and low density. They have been utilized not only as anode materials but also conductive additives (for silicon powder based composite anodes) and as the current collector. Lithium storage thermodynamics depends on the specific structural features of the CNTs, particularly the diameter of the tubes and extent of defects.
Graphene Materials
Graphene materials, including reduced graphene oxide, are known for high electrical conductivity, specific surface area and chemical stability. As a result, they provide high specific power and long cycle life to batteries that use them as anode materials. Graphene materials can be added via the form of porous frameworks such aerogels, foams and sponges. Graphene is highly promising and can provide a large gravimetric capacity (>1000 mA h g−1). The ion-storage properties of graphene materials vary largely along with lateral dimensions, layer number and defects. However, these properties can be controlled through different synthesis techniques.
Carbon Black
In composite anodes, carbon black is used alongside anode active materials to increase electronic conductivity and suppress excessive solid-electrolyte interphase formation. The structure of carbon black has more orientations and a larger lattice spacing than graphite. As a result, Li+ intercalation can occur more quickly, enabling faster charging capabilities.
Examples of carbon-based anode materials
Browse Carbon-based Anode Active Materials
Metal-Based Anode Materials
Metal-based anode materials play a crucial role in advancing high-performance rechargeable batteries. These materials include both elemental metals and metal-containing compounds, offering unique electrochemical behaviors such as high theoretical capacities, multi-electron redox activity, and in some cases, exceptional cycling stability. Their use spans a variety of battery chemistries, including lithium-ion, sodium-ion, and solid-state systems. Their versatility makes them a key component in the development of next-generation energy storage solutions.
Metallic Lithium
Metallic lithium (Li0) has ultra-high theoretical specific capacity (~3860 mAh/g), making it the most ideal anode material for lithium-based batteries. However, it is highly reactive with common electrolytes. As a result, it has a poor cycle life and has issues with safety. Solid state batteries aim to tackle the issues of metallic lithium safety by using solid electrolytes to protect the cell from lithium plating and dendrite formation. Protective coatings have also been used as a method to preserve battery life.
Lithium Titanium Oxide (LTO)
Lithium titanium oxide (LTO) is used instead of carbon materials like graphite as the anode active material due its high intercalation potential, great rate capability, and high Li+ diffusion. It has good cycle stability as there is very little change in the volume of the crystal when lithium ions are intercalated or not. LTO has a high-power density making it particularly attractive properties for electric vehicles batteries.
Transition Metal Oxides (TMOs)
Transition metal oxides (TMOs) have been considered as promising anode materials for rechargeable Li-ion batteries (LIBs) with high theoretical capacity compared to that of conventional graphite. Many transition metal oxides possess the ability to store lithium or sodium via conversion reactions. Conversion-type anodes generally exhibit higher specific capacities. They can undergo a transformation into metallic clusters and Li2O/Na2O during the process:
MOy + 2y A+ + 2y e− ↔ y A2O + x M
There are a huge range of metal oxides available for use as conversion-type alkali metal ion storage. As a result, tailored design is possible based on desired capacity, voltage, and stability. Many transition metals are able to access multiple oxidation states, enabling multi-electron reactions per formula unit. TMOs often require conductive additives or nanostructuring to improve low electrical conductivity. They also suffer from significant volume changes as a result of conversion reactions.
Transition Metal Dichalcogenides (TMDs)
Transition metal dichalcogenides (TMDs) have also been used as conversion-type anode materials in batteries. Compared to transition metal oxides, TMDs have better electrical conductivity and electrochemical reversibility. Many have a 2D layered structure which can provide a convenient channel for ion diffusion. The performance of TMDs electrochemical storage is impacted by the SEI film formed during the discharging/charging process as well as volume variation and low ion diffusion rates. As a result, TMDs are often used in composites alongside carbon when applied as anodes.
Metallic Additives
Metallic additives are used within anodes in order to increase energy density. Silver nanoparticles have been used to enhance graphite anodes by lowering the negative/positive capacity ratio and increase the charging voltage. Metallic seeds promote homogeneous lithium plating and reduce the risk of dendrite formation. Certain metals (Co, Ni) act as electrocatalysts, enhancing interfacial kinetics or assisting in solid-electrolyte interphase (SEI) formation.
Examples of metal-based anode materials
Browse Metal-based Anode Active Materials
Alloy-based Materials
Alloy-based anodes offer a promising route for high-capacity energy storage by using a different mechanism from traditional intercalation or conversion-type materials. Instead of simply inserting lithium ions between host layers (as in graphite) or transforming into metal oxides (as in conversion-type anodes), alloy-based materials undergo a direct chemical reaction with alkali metal ions to form alloys (AxM).
Several main-group elements, including silicon (Si), antimony (Sb), germanium (Ge), bismuth (Bi), and tin (Sn), can form such alloys and offer high specific capacities. For example, tin undergoes the following reaction:
Sn + x A+ + x e− ↔ AySn
These alloying reactions deliver significantly higher capacities than conventional graphite but are typically associated with substantial volumetric expansion. This expansion and subsequent contraction during lithiation/delithiation cycles can cause mechanical stress, cracking, and electrical disconnection, resulting in rapid capacity fade.
To mitigate these issues, engineering strategies such as nanostructuring, forming composites with conductive carbon materials, and using flexible polymeric binders are widely employed. These approaches help to accommodate volume changes, maintain structural integrity, and prolong the cycling life of alloy-based anodes.
Alloying elements can generally be categorized into two groups based on their chemical nature: metalloids (e.g., Si, Sb, Ge) and post-transition metals (e.g., Sn, Bi), both of which contribute uniquely to the development of high-performance batteries.
Metalloid-Based Anode Materials
Silicon
Silicon nanopowder is widely explored as an anode material for Li-ion batteries due to its high theoretical capacity of 3579 mAh/g (Li15Si4). Si-based anodes deliver high energy density, limit growth of interphase, and are free of Li dendrites in solid state batteries. However, Si undergoes a significant volumetric expansion during lithiation, leading to cracking, pulverization, and poor long-term electrochemical performance. Nanosizing helps mitigate the large volume expansion (~400%) during lithiation, improving structural integrity and cycle life.
Si + x Li⁺ + x e⁻ → LixSi (e.g., Li15Si4)
Black Phosphorous
Black phosphorous (BP) can alloy with lithium to form Li3P, offering a theoretical specific capacity of ~2596 mAh/g, far surpassing traditional graphite (~372 mAh/g). BP has a 2D puckered-layered structure which provides short ion diffusion paths, fast ion intercalation/deintercalation and supports high-rate performance. Like silicon, BP undergoes significant volume expansion upon lithiation. Nanostructuring, composite formation, and encapsulation strategies are commonly used to overcome this issue.
Organic Anode Materials
Organic anode materials can be split into four main classes: organic carbonyl compounds, covalent organic frameworks (COFs), metal-organic frameworks (MOFs), and organic compounds with nitrogen-containing groups. They have recently attracted attention as anode materials due to their low cost, high theoretical capacity, structural diversity, environmental friendliness, and facile synthesis. Organic compounds with electroactive functional groups or moieties, either a polymer or small organic molecule, can undergo an electron transfer through a reversible electrochemical redox reaction. Organic molecules can operate in aqueous, solid-state, or organic electrolyte systems, providing compatibility with a wide range of battery chemistries. They enable modular molecular design, allowing tailoring of redox potential, solubility, and stability through synthetic chemistry.
Significant issues, such as poor electronic conductivity, low discharge voltage, and undesired dissolution of active organic anode material into typical organic electrolytes are currently being addressed.
Learn More
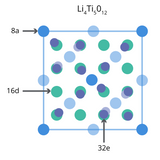 What is an LTO Battery?
What is an LTO Battery?
An LTO battery is named after its key component, lithium titanium oxide (LTO) powder. The material is also referred to as lithium titanate with the chemical formula Li4Ti5O12. Unlike most lithium-ion battery materials it is used as the anode active material.
Learn more...A solid electrolyte interphase (SEI) forms on the negative electrode in lithium-ion batteries (LIBs) due to the decomposition of electrolyte. The decomposition by-products build up on the surface of the anode and form an independent phase of material, different to the electrode and electrolyte.
Read more...
References
- Structural Engineering of Anode Materials for Low-Temperature Lithium-Ion..., Wang, G. et al., Nano-micro letters (2024)
- A review on applications and challenges of carbon..., Tong, Z. et al., Carbon Energy (2024)
- A comprehensive review of the use of porous..., Okhay, O. et al., Journal of Energy Storage (2024)
- Carbon materials for ion-intercalation involved rechargeable battery technologies, Wang, G. et al., Chem. Soc. Rev. (2021)
- Lithium-Ion Battery Anodes Based on Graphite: Performance Enhancement..., Beden. S. A. J. et al., Revue des composites et des matériaux avancés (2025)
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer







