What are Metal Organic Frameworks (MOFs)?

Jump to: Types of Metal Organic Framework | MOFs Structure and Porosity | Metal Nodes and Clusters | MOF Ligands | Coordination Bonds
Metal-organic frameworks (MOFs) are a class of 3D materials that are made up of metals connected by organic MOF ligands. Think of the metals and organic compounds as building blocks that form these MOF structures.
These structures can come in a variety of shapes and sizes, ranging from 1D to 3D, but they often create a repeating pattern. For this reason, MOFs are often described as reticular (gridlike). MOFs are like an atomic scale version of the magnetic ball and stick toys where the metals act as connectors (the balls) and the organic compounds act as linkers (the sticks).
Metal-organic frameworks are also known as:
- Hybrid organic−inorganic materials
- Metal-organic polymers
- Coordination polymers
- Organic zeolite analogs
MOFs have been revolutionary in the field of chemistry, so much so that on October 2025, Professor Susumu Kitagawa, Professor Richard Robson and Professor Omar Yaghi won the nobel prize in Chemistry for the development of metal-organic frameworks. There are 90,000+ different MOFs out there and many more to be discovered! MOF-5 is one of the most famous as it has an extremely high surface area of 2200 m2/cm3 which is approximately 15 times that of human lungs.
Types of Metal Organic Framework
As there are over 90,000 different metal organic frameworks (MOFs), these are often grouped into different families. MOFs are named in a variety of ways but all have a lettered code with a number following it (eg. ZIF-8). Some are named based on their features. For example, MOFs with similar structures but with slightly different organic components are described as isoreticular ("iso" = same) MOFs so they are named IRMOFs. Other MOFs are name based on where they were discovered (eg. UiO - University of Oslo). The number that follows the lettered code indicates the specific MOF within the family. Some examples of different MOF families include:
- Northwestern University (NU)
- Pohang University of Science and Technology (POST-n)
- Dresden University of Technology (DUT-n family)
- University of Nottingham (NOTT-n)
- Hong Kong University of Science and Technology (HKUST-n) e.g. HKUST-1
- Christian-Albrechts-University (CAU-n family)
Browse Metal Organic Framework Products
Browse metal organic frameworks
MOFs Structure and Porosity
Metal organic frameworks are strong, porous materials part of the porous organic frameworks family. Metal ions or cluster acts as nodes, which are linked together by "organic linkers" creating 3D frameworks. The spaces within the connected metals and organic linkers are called pores. These pores can be very small, in the range of a few angstroms (1 angstrom = 0.1 nanometers), to relatively large, exceeding several nanometers in diameter. Pore size is controlled by the size of the linker compound used and how it coordinates with the metal center.
Some MOFs contain multiple different organic compounds, forming different sized pores within the structure. This tunability and building block nature means that, unlike other porous materials, MOFs can be designed for specific applications like gas separation. This is done by selecting specific linkers to get the desired pore size. Tuning the different chemical properties of both the selected metal and organic compound also brings advantages.
MOFs
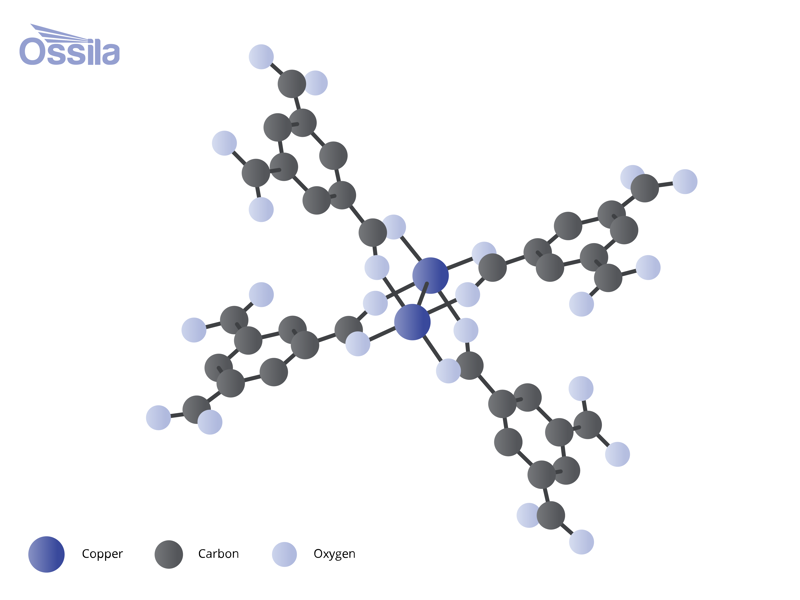
Metal Nodes and Clusters
The metals nodes within metal organic frameworks can be just one atom or a cluster of atoms. They are also referred to as metal centers or inorganic centers. Multiple organic linker compounds can bond to single metal center. Clusters of metal atoms, often connected by oxygen, also act as connectors between organic linkers. Examples of metal clusters used in MOFs include:
- M3O (M = Al, Fe, Cr)
- M4O (M = Zn)
- M6O4(OH)4 (M = Zr, Hf, Ce)
- M8O8(OH)4 (M = Ti)
Metal clusters are also referred to as secondary building units (SBUs). Strong bonds within metal clusters gives them specific geometries. The atoms are precisely arranged with designated sites for connectivity. This arrangement allows coordination bonds with linkers to occur in predictable and repeating patterns.
The type of metal centers used brings their own chemical properties to the metal organic framework. Here are a few examples:
| Metal | Properties |
| Cu, Ni, Co | Catalytic |
| Fe | Magnetic, catalytic, biocompatible |
| Ti | Redox active, photochemical, biocompatible |
| Mn | Catalytic, high capacitance |
| Zn | Catalytic, biocompatible |
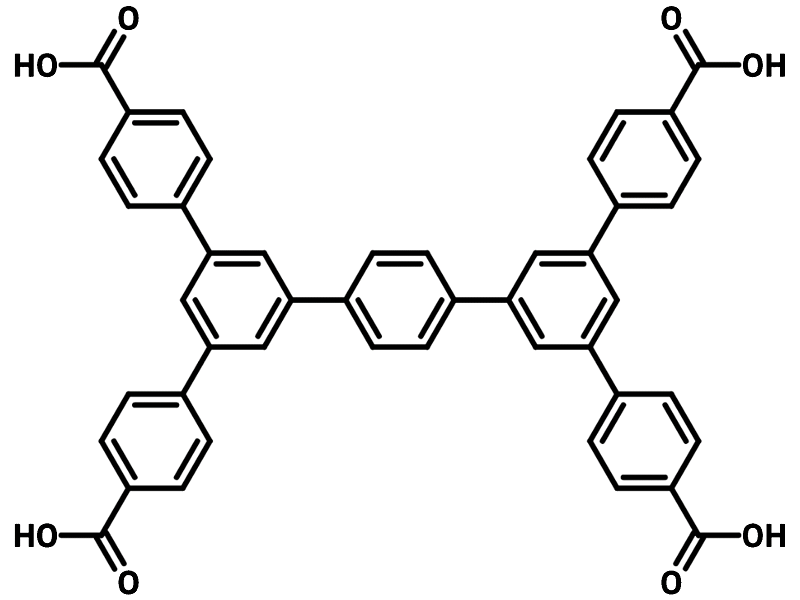
MOF Ligands
MOF ligands (also known as organic linkers) connect the metal centers. For an organic compound to be a ligand, it must have at least two coordinating sites. These sites contain electronegative atoms such as oxygen or nitrogen. Coordinating functional groups include:
- Boronic acid
- Carboxylic acid
- Phosphonic acid
- Sulfonic acid
- Amine
- Heterocyclic amine
Electrons from the electronegative atoms are shared with metal centers. This is called a coordination bond and will be discussed in more detail below. Organic linkers are described as ditopic, tritopic, tetratopic, or multitopic depending on the number of coordinating functional groups:
The properties of the linker compound are also incorporated into metal-organic frameworks. The structure, length, ratio, and types of functional groups in a linker affect the size, shape, and internal surface properties of a MOF. Organic linker compounds can also contain functional groups that are not involved in coordination bonds with the metal centers. These functional groups bring different properties to the MOF. They can even be modified after MOF synthesis to incorporate more properties for suitable different applications.
MOF Ligands
Coordination Bonds
In metal organic frameworks, coordination bonds form between the metal and organic ligand. Atoms within organic compounds such as oxygen or nitrogen donate lone pairs of electrons to the metal center. It is also referred to as a dative covalent bond as both electrons in the metal-ligand bond originate from the same atom (ligand). Coordination bonding is strong but mostly reversible. You can break the bonds by changing the pH of the MOF in solution.
The number of ligands attached to the metal center is referred to as the coordination number. This can vary depending on the size, charge, and electron configuration of the metal ion as well as the size and electron-donating ability of the ligands.
Learn More
Metal-organic frameworks (MOFs) are made by connecting metal centers with organic linkers through coordination bonds. The process of creating MOFs plays a crucial role in crystal structure formation. This determines their properties and how well they perform in various applications.
Learn more... What is Surface Energy? Formula & Definition
What is Surface Energy? Formula & Definition
Surface free energy is a measure of the excess energy present at the surface of a material, in comparison to at its bulk. It can be used to describe wetting and adhesion between materials.
Read more...References
- Furukawa, H. et al. (2013). The Chemistry and Applications of Metal-Organic Frameworks. Science.341. 10.1126/science.1230444
- Li, C. et al. (2022). Metal Centers and Organic Ligands Determine Electrochemistry of Metal–Organic Frameworks, Small, 18. doi:10.1002/smll.202106607
- Yang, D. et al. (2020). The Surface Chemistry of Metal Oxide Clusters: From Metal–Organic Frameworks to Minerals, ACS Cent. Sci., 6. doi:10.1021/acscentsci.0c00803
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer