PCE12, better solubility and film morphology than PCE11
Paired with PC71BM to achieve high device performances
Specifications | Pricing and Options | MSDS | Literature and Reviews
Compared to PffBT4T-2OD (PCE11), PffBT4T-C9C13 has larger side chains which help promote better solubility and film morphology. When using PffBT4T-C9C13 as a polymer donor and PC71BM as electron acceptor (with trimethylbenzene (TMB) as host solvent), a higher device performance of 11.7% was achieved [2].
Due to its broader absorption of the solar spectrum in the visible light region (with an absorption edge at about 800 nm), PffBT4T-C9C13 is also ideal for use as a donor material for all-polymer solar cells and NFA-polymer solar cells
Additionally, its higher solubility means that PffBT4T-C9C13 can be processed with non-halogenated solvents. There are great opportunities with this polymer for inkjet printing on an industrial scale - an environment-friendly approach for alternative renewable energy.
General Information
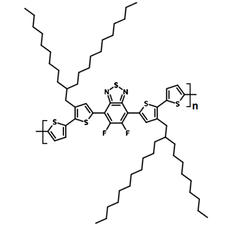
| Full name | Poly[(5,6-difluoro-2,1,3-benzothiadiazol-4,7-diyl)-alt-(3,3’’’-di(2-nonyltridecyl)-2,2’;5’,2’’;5’’,2’’’-quaterthiophen-5,5’’’-diyl)] |
| Synonyms | PffBT4T-C9C13, PCE12 |
| Chemical formula | (C66H97F2N2S5)n |
| CAS number | 2001095-79-8 |
| HOMO / LUMO | HOMO = -5.34 eV LUMO = -3.69 eV [1] |
| Solubility/processing solvents | Trimethylbenzene (TMB), chlorobenzene |
| Classification / Family | Benzothiadiazole, Fluorinated benzothiadiazole, Heterocyclic five-membered ring, Organic semiconducting materials, Low band gap polymers, Organic photovoltaics, All polymer solar cells, NFA-polymer solar cells |
Chemical Structure
Pricing
| Batch | Quantity | Price |
| M2083A1-3 | 100 mg | £440 |
| M2083A1-3 | 250 mg | £1000 |
| M2083A2-3 | 500 mg | £1800 |
| M2083A2-3 | 1 g | £3200 |
| M2083A | 5 - 10 g* | Please enquire |
*For order quantities of 5-10 grams, the lead time is 4-6 weeks.
Batch details
| Batch number | MW | MN | PDI | Stock Info |
| M2083A1 | 123,796 | 73,818 | 1.68 | Discontinued |
| M2083A2 | 136,000 | 78,000 | 1.71 | Discontinued |
| M2083A3 | 118,000 | 73,000 | 1.61 | Discontinued |
| M2083A4 | 143,000 | 79,000 | 1.81 | In Stock |
MSDS Documentation
Literature and Reviews
- Towards a bright future: polymer solar cells with power conversion efficiencies over 10%, Z Hu et al., Sci. China Chem, 60 (5), 571-582 (2017); doi: 10.1007/s11426-016-0424-9.
- Efficient organic solar cells processed from hydrocarbon solvents, J. Zhao et al, Nat. Energy 1, 15027 (2016); doi:10.1038/nenergy.2015.27.
