PCE11 (PffBT4T-2OD)
CAS Number 1644164-62-4
Chemistry Building Blocks, Materials, OPV Polymers, Semiconducting PolymersPCE11, highly efficient semiconducting polymer for OPVs
Excellent hole transport mobilities and available online for fast, secure dispatch
Specifications | MSDS | Literature and Reviews
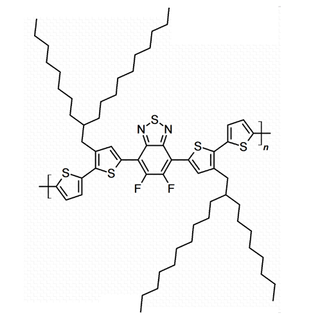
PffBT4T-2OD (PCE11), CAS number 1644164-62-4, is a low band-gap (1.65 eV) semiconducting polymer for organic photovoltaics (OPVs), which has reached power conversion efficiencies (PCEs) approaching 11% [1]. These efficiencies are a result of the high crystallinity of the polymer, providing excellent hole transport mobilities on the order of 10-2 cm2V-1s-1, and the ability to use a thick active layer, resulting in improved light absorption.
The size and position of the alkyl chains of PffBT4T-2OD are critical to its temperature dependant aggregation properties, enabling control over the aggregation and crystallization of the polymer to produce an efficient donor:acceptor film morphology.
PCE11 (PffBT4T-2OD) from Ossila was used in the high-impact paper (IF 14.92), High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor, S. Holliday et a., Nat. Commun., 11585 (2016); DOI: 10.1038/ncomms11585.
Polymer PCE11 was targeted by reacting 4,7-bis(5-bromo-4-(2-octyldodecyl)thiophen-2-yl)-5,6-difluorobenzo[c][1,2,5]-thiadiazole with 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene engaging Stille Coupling reaction.
![pce11-pffbt4t-2od-synthesis 4,7-bis(5-bromo-4-(2-octyldodecyl)thiophen-2-yl)-5,6-difluorobenzo[c][1,2,5]-thiadiazole with 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene](https://www.ossila.com/cdn/shop/files/pce11-PffBT4T-2OD-synthesis.jpg?v=1718781996)
For 5 - 10 grams order quantity please enquire, the lead time is 4-6 weeks.
General Information
| Full name | Poly[(5,6-difluoro-2,1,3-benzothiadiazol-4,7-diyl)-alt-(3,3’’’-di(2-octyldodecyl)-2,2’;5’,2’’;5’’,2’’’-quaterthiophen-5,5’’’-diyl)] |
| Synonyms | PffBT4T-2OD |
| Chemical formula | (C62H88F2N2S5)n |
| CAS number | 1644164-62-4 |
| HOMO / LUMO | HOMO = -5.34 eV, LUMO = -3.69 eV [1] |
| Soluble in | Chloroform, chlorobenzene, dichlorobenzene |
| Recommended Processing Solvents at 10mg/ml | Chlorobenzene at elevated temperature ca. 110 °C |
| Classification / Family | Benzothiadiazole, Fluorinated benzothiadiazole, Heterocyclic five-membered ring, Organic semiconducting materials, Low band gap polymers, Organic Photovoltaics, Polymer Solar Cells |
Batch Details
| Batch number | MW | MN | PDI | Stock Info |
| M303 | 112,707 | 55,674 | 2.02 | In stock |
Chemical Structure
Device Structure(s)
The structure of the high-performance (10 - 11%) devices was:
ITO / ZnO / PffBT4T-2OD:PC70BM (200 – 300 nm) / MoO3 or V2O5 (20 nm) / Al (100 nm)
PffBT4T-2OD:PC70BM solution details:
- Blend ratio: 1:1.2,
- Polymer concentration: 9 mg/ml,
- Solvent: 1:1 blend of chlorobenzene and dichlorobenzene,
- Additive: 3% diiodooctane,
- Heating: 85°C for dissolution,
It is important to note that this solution (and the substrate being deposited onto) must be heated for spin casting, with the ideal temperature being 60 – 80°C. It is reported that a solution and substrate pre-heating temperature of 110°C should be used to allow for cooling that will occur before deposition.
MSDS Documentation
PCE11 (PffBT4T-2OD) MSDS sheet
Literature and Reviews
- Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells, Y. Liu, et al., Nat. Comm., 5, 5293 (2014)
- High-efficiency non-fullerene organic solar cells enabled by a difluorobenzothiadiazole-based donor polymer combined with a properly matched small molecule acceptor, J. Zhao et al., Energy Environ. Sci., 8, 520-525 (2015)
