3-BPIC-F
CAS Number 3038707-50-2
Charge Transport Layer Materials, OLED Materials, Perovskite Interface Materials, Perovskite Materials,Small Self-Assembled Monolayer Molecule for High Efficiency Solar Cells
Bipodal hole transport or extraction layer for NFA-polymer solar cells and p-i-n perovskite solar cells
Product Information | MSDS | Literature and Reviews
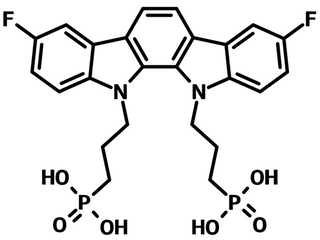
3-BPIC-F is a self-assembled monolayer (SAM) material that consists of a 3,8-difluoroindolo[2,3-a]carbazole terminal functional group with two phosphonic acid anchors and propanyl spacers that join the anchors to the electron rich terminal. 3-BPIC-F is the fluorinated derivative of 3-BPIC. The incorporation of electron-withdrawing fluorine atoms enables the ITO/3-BPIC-F substrate with an enlarged work function (WF), showing a deeper highest occupied molecular orbital (HOMO) energy level and a larger dipole moment while compared to those of 3-BPIC.
As the hole selective contact, the self-assembled 3-BPIC-F monolayer not only improves the hole extraction rate within the device but also lowers the interfacial impedance to reduce nonradiative recombination at the interface. Moreover, organic solar cells based on 3-BPIC-F as the interface between the anode and photoactive layer exhibit enhanced operational stability compared to those based on PEDOT:PSS as the hole extraction layer (HEL). Record high device power conversion efficiency (PCE) of 19.71% was achieved for polymeric organic non-fullerene solar cell based on PM6:L8-BO:BTP-ec9 (1:1:0.3 w/w) as the active layer, and 3-BPIC-F as the SAM interface with a simplified structure of ITO/3-BPIC-F/PM6:L8-BO:BTP-ec9/PNDIT-F3N/Ag.
Serving as hole selective contact for organic solar cells and perovskite solar cells, 3-BPIC-F is an alternative to PEDOT:PSS with superior performance with the convenience of solution deposition at low concentration, i.e. 1 mM.
Solution Processing Procedure
Typical processing solvents: Ethanol, methanol, IPA, DMF, THF
Typical concentration: 1 mM - 1.0 mg/ml
Typical processing procedure: 3-BPIC-F is dissolved in ethanol at the concentration of 0.9 mg/mL. The ITO/SAM monolayer film is deposited by spin-coating the solution on the top of the ITO glass substrate at 3000 rpm for 20 s and then annealed in air for 10 min. The ITO/SAM substrate is dynamically washed with the same solution at 5000 rpm for 20 s (DOI: 10.1021/jacs.4c03917).
General Information
| CAS Number | 3038707-50-2 |
|---|---|
| Chemical Formula | C24H24F2N2O6P2 |
| Molecular Weight | 536.4 g/mol |
| Absorption | λmax (n.a.) |
| Fluorescence | λem (n.a.) |
| HOMO/LUMO | HOMO = 5.39 eV, LUMO = 1.16 eV |
| Synonyms | IDCz-2F, (3,8-Difluoroindolo[2,3-a]carbazole-11,12-diyl)bis(propane-3,1-diyl)diphosphonic acid |
| Classification or Family | Indolo[2,3-a]carbazole derivatives, Self-assembly monolayers, Hole transport layer, Hole extraction layer, p-i-n Perovskite solar cells, Organic photovoltaics |
Product Details
| Purity | > 98% (HPLC) |
|---|---|
| Melting Point | N/A |
| Appearance | White powder/crystals |
Chemical Structure
MSDS Documentation
Literature and Reviews
- H. Liu et al. (2024); Dipole Moments Regulation of Biphosphonic Acid Molecules for Self-assembled Monolayers Boosts the Efficiency of Organic Solar Cells Exceeding 19.7%, J. Am. Chem. Soc., 146 (20), 14287–14296; DOI: 10.1021/jacs.4c03917.
- J. Wu et al. (2024); Bisphosphonate-Anchored Self-Assembled Molecules with Larger Dipole Moments for Efficient Inverted Perovskite Solar Cells with Excellent Stability, Adv. Mater., 36 (28), 2401537; DOI: 10.1002/adma.202401537.
