Anode vs Cathode: What is the Difference?

The electrodes, anodes and cathodes, are important components of electrical devices such as batteries. When an electric current is applied, these electrodes facilitate the transfer of electrons through the system. In batteries, the accumulation or depletion of electrons at an electrode surface is electrochemically coupled with ion migration, facilitating energy storage and conversion.
Comparing anode vs cathode: the distinction between the two is often confusing. They are commonly described as the positive and negative electrodes, but this definition is not always applicable. An electrode can be negatively or positively charged depending on the state of the battery. The components of a battery are conventionally named based on their roles during a specific state of operation, typically during discharge.
What is a Cathode?
In traditional electrochemical terminology, the cathode is defined as the electrode where reduction (the gaining of electrons) occurs. When current flows, the cathode potential becomes lower than its equilibrium (rest) potential.
In terms of electrochemical cells, this means that the cathode can be positive or negative depending on the type of electrochemical cell:
- In galvanic cells (which generate electricity from spontaneous reactions), the cathode is positive. Electrons flow through the external circuit to the cathode, where positive ions in the electrolyte gain electrons and are reduced.
- In electrolytic cells (which use electricity to drive non-spontaneous reactions), the cathode is negative. An external power source pushes electrons toward the cathode, where they are accepted by ions, driving reduction.
What is an Anode?
In tradition electrochemical terminology, the anode is defined as the electrode where oxidation (the loss of electrons) occurs. When current flows, the anode potential rises above its equilibrium (rest) potential.
Therefore, like the cathode, the anode is negative or positive depending on the type of cell.
- In galvanic cells, the anode is negative. It is the source of electrons that flow through the external circuit to the cathode. The material at the anode loses electrons and is oxidized.
- In electrolytic cells, the anode is positive. The external voltage pulls electrons away from the anode, lowering its electron density and causing oxidation to occur at its surface.
Can they be labeled as positive or negative?
When defining anode vs cathode, it is more accurate to refer to electrodes based on their electrical potential rather than by assuming fixed charges. The positive electrode is the one with a higher potential, and the negative electrode has a lower potential. The anode and cathode designations relate to the type of reaction occurring (oxidation or reduction), not to their static charge.
However, charge labels (positive or negative) can still be useful, as long as the type of cell and its operating state (charging vs. discharging) are clearly defined. Using charge-based labels without context can lead to confusion, but within a specific system, they help describe electron flow, direction of ion migration, and circuit configuration. So while not universal, charge can be a valid and practical way to label electrodes.
Defining Current
Defining anode vs cathode as negative vs positive, or as the source vs sink of a current, depends on your definition of current itself. Current can describe the flow of positive or negative charge:
- Conventional current describes the flow of positive charge. This is despite the fact electrons constitute the actual electrical flow.
- Electron current describes the actual flow of negative electrons.
The flow direction of electrons and positive ions depends on the state of the battery. Thus, it is important to describe the system further when defining the difference between an anode and a cathode.
How does this effect the definitions?
It is important to specify the exact operating conditions of the cell. Electron and ion flow directions change depending on the state of the battery (charging or discharging), so it is important in defining electrode behavior.
Electrodes in Action
While many principles discussed here apply broadly to rechargeable batteries, this section focuses primarily on the behavior of electrodes in lithium-ion batteries, the most widely used type in modern electronic devices. Importantly, electrode naming conventions, such as anode and cathode, are typically based on the battery’s behavior during discharge, when it is supplying power. In this state, the anode is the source of electrons (oxidation), and the cathode is where electrons are accepted (reduction). These names remain fixed even though the roles of the electrodes in terms of ion and electron flow reverse during charging. This convention helps maintain consistency in technical language, but it can be a source of confusion when analyzing charging behavior.
Discharging
During discharge, the battery spontaneously generates a potential difference as the system moves toward chemical equilibrium. Now, the anode becomes the electrode with a higher potential, and the cathode has a lower potential, relative to each other under load conditions.
Electrons are released from the anode and travel through the external circuit, providing usable electric power. Simultaneously, ions stored at the anode migrate through the electrolyte back to the cathode. At the cathode, these ions recombine with electrons to complete the reduction reaction.
The electromotive force (EMF) or cell voltage of the battery during discharge reflects the difference in electrode potentials under operating conditions. This potential difference is the driving force that powers electronic devices.
Once fully discharged, the chemical energy has been converted into electrical energy, and the materials return to their lower-energy states. This state can be reversed again through recharging.
Charging
During charging, an external voltage is applied to reverse the natural electrochemical flow of the battery. This voltage must be greater than the difference in electrode potentials between the cathode and anode to drive the reaction in reverse.
In this state, the cathode (which has a higher potential) releases positive ions and electrons. The ions travel through the electrolyte, while the electrons are forced through the external circuit toward the anode (which has a lower potential during charging).
At the anode, these ions are stored via intercalation, plating, or surface reactions depending on the battery chemistry. Electrons accumulate at the anode as well but are prevented from returning to the cathode directly by the electrolyte, which only allows ionic (not electronic) conductivity.
The difference in electrical potential between the two electrodes under charging conditions determines how much external energy is needed to store charge. In rechargeable batteries, this process is repeatable across many cycles.
Is the type of battery important?
The type of battery chemistry plays a significant role in how anodes vs cathodes function, as well as the charging or discharging state. Different battery systems use different materials and electrochemical reactions, which directly influence the behavior, performance, and definition of electrodes.
Lithium-Ion Batteries
These are intercalation-type batteries, where lithium ions move between the anode (typically graphite) and the cathode (commonly layered metal oxides like LiCoO2, LiFePO4, or NMC). For conventional lithium-ion batteries, in the discharging state the battery behaves as a galvanic cell and when charging it functions as an electrolytic cell.
- During discharge, the cathode is the positive electrode, accepting lithium ions and electrons.
- During charge, the roles reverse in terms of ion flow, but the cathode remains the site of reduction.
Sodium-Ion Batteries
Sodium-ion batteries operate similarly to lithium-ion systems, using intercalation reactions. However, due to sodium's larger ionic radius, the materials and design constraints differ. Common cathodes include layered oxides and Prussian blue analogs. These batteries are attractive for grid storage due to the abundance of sodium.
Lead-Acid Batteries
In lead-acid batteries, the cathode is made of lead dioxide (PbO2), and the anode is sponge lead (Pb). The electrolyte is sulfuric acid.
- During discharge, PbO₂ is reduced, and Pb is oxidized.
- Both electrodes convert to lead sulfate (PbSO4), making it a conversion-type battery rather than intercalation.
Nickel Batteries
These systems use nickel oxyhydroxide (NiOOH) as the cathode material and either cadmium or a hydrogen-absorbing alloy as the anode.
- Redox reactions involve the conversion of nickel and cadmium species rather than ion intercalation.
Lithium-Sulfur Batteries
In these high-energy-density batteries, sulfur acts as the cathode and lithium metal as the anode. The cathode undergoes complex multi-electron reduction reactions, forming lithium polysulfides. This is a conversion-type system with significant capacity but challenging cycling stability.
Solid-State Batteries
Solid-state batteries replace liquid electrolytes with solid ones, allowing for the use of lithium metal anodes and high-voltage cathodes, potentially improving energy density and safety.
Emerging Chemistries
Other emerging systems (e.g. lithium-air, magnesium-ion, aluminum-ion) bring their own unique definitions and behaviors for electrodes due to fundamentally different chemistries.
Cathode Materials
For intercalation-based batteries, such as lithium-ion batteries, the cathode supplies the positive ions that allow for intercalation with the anode.
The battery materials used influence the intercalation process. Lithium-ion batteries use lithium ions (Li+), while sodium-ion batteries use sodium ions (Na+). The chemistry and structure of the cathode is selected to enhance the discharge rates and overall capacity of the battery.
The intercalation process is dependent on the cathode and is the foundation for how batteries work. As the source of positive ions, cathodes are typically the most complicated and important element of a battery.
While research into all the components of a battery can push the experimental capacity closer to the theoretical, the cathode requires the most development. The cathode material can be designed to tackle different tasks with certain chemistries offering better cycle life in exchange for power output, and vice versa.
Examples of cathode active materials
Anode Materials
In intercalation-based batteries, the anode is the intercalation point for positive ions.
Typically, the anode requires a porous structure so intercalation can occur, and it must be electronically conductive. Other material qualities considered include thermal and electrical isometry. An anode that expands or contracts during intercalation or heating can cause battery failure.
Carbon nanotubes and graphene based materials make excellent anode active materials due to their high electron conductivity, stability and low weights.
Examples of anode active materials
Other Applications Outside of Batteries
Electrodes are essential in any electrical device that requires the injection, collection, or regulation of charge flow. They enable the release, movement, or storage of energy, playing a critical role well beyond batteries, including:
- Photovoltaic devices (like solar cells) - electrodes collect the charge carriers (electrons and holes) generated when sunlight excites the photoactive material, enabling the conversion of light into electricity.
- Electrolysis systems - electrodes drive chemical reactions such as splitting water into hydrogen and oxygen by applying an external voltage.
- Fuel cells - electrodes facilitate redox reactions between a fuel (hydrogen) and an oxidant to generate electricity.
- Supercapacitors - electrodes store energy through electrostatic charge separation at the interface between the electrode and electrolyte.
- Sensors and medical devices - electrodes are used to detect electrical signals or deliver controlled currents for stimulation or diagnostics.
Summary
Cathodes and anodes are key parts of a battery. While often labeled as either positive or negative, these terms do not fully describe their roles. The direction of current, either as positive charge flow or actual electron flow, also matters. Whether the battery is charging or discharging determines which electrode supplies the flow of electrons. In both processes, a flow of negative electrons and positive ions occurs at the same time, in the same direction. Therefore, the positive and negative terminals are not the same in all cases.
Related Products
Learn More
 An Introduction to Batteries
An Introduction to Batteries
Typically, batteries work by a process known as intercalation. This process occurs across the battery components. Most batteries consist of the same components.
Read more...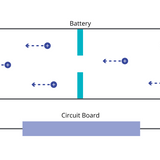 Lithium-Ion Battery Components and Working Principle
Lithium-Ion Battery Components and Working Principle
Lithium-ion batteries use the reversible lithium intercalation reaction. The battery has several important components to enable this intercalation.
Read more...Contributing Authors
Written by
Scientific Writer
Edited by
Application Scientist
Diagrams by
Graphic Designer









