Small SAM Molecule for High Efficiency Solar Cells
Hole transport or extraction layer for NFA-polymer solar cells and p-i-n perovskite solar cells
Specifications | MSDS | Literature and Reviews
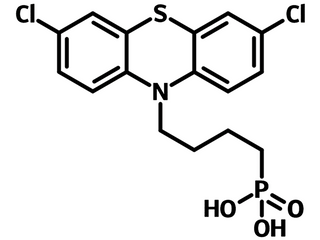
TDPA-Cl is a self-assembled monolayer material with similar functionality to Br-2EPT bearing the same terminal functional group of 10H-phenothiazine and head anchor phosphonic acid to the surface. The differences are that the terminal group is 3,7-dichlorinated instead of brominated, and the spacer has a length of four-carbon alkyl chain rather than two.
By engaging chlorinated phenothiazine as the headgroup, TDPA-Cl has proven to be a universally applicable self-assembled monolayer material that can be used as effective hole selective contact layer for both non-fullerene acceptor (NFA) organic solar cells and perovskite solar cells. The synergistic effect of electronegativity, together with the empty 3d orbits of chlorine atoms, can lead to increased intramolecular and intermolecular interactions, down-shifting the molecular energy levels.
As a hole selective contact, TDPA-Cl demonstrated superior performance to those with PEDOT:PSS in the PM6:Y6-based organic solar cells. The TDPA-Cl-based devices delivered a better performance of 22.4% than the PTAA-based devices (20.8%) with improved processability and reproducibility in perovskite solar cells, and an efficiency of 17.4% in the D18:Y6 based organic solar cells.
Serving as hole selective contact for organic solar cells and perovskite solar cells, TDPA-Cl is an alternative to PEDOT:PSS with superior performance with the convenience of solution deposition at low concentration, i.e. 1 mM.
Solution Processing Procedure
Typical processing solvents: ethanol, methanol, IPA, DMF
Typical concentration: 1 mM (0.4 mg/ml) or 1.0 mg/ml (1 mg TDPA-Cl is dissolved in 1 ml methanol)
Typical processing procedure: 40 uL of TDPA-Cl in methanol solution (1.0 mg/ml) is deposited onto the center of the substrate surface and spin-coated for 30 s at the speed of 3000 rpm. The coated film on the substrate is then annealed for 10 min at 80 ℃ (DOI: 10.1021/acs.langmuir.3c03610).
General Information
| CAS Number | N/A |
|---|---|
| Chemical Formula | C16H16Cl2NO3PS |
| Molecular Weight | 404.25 g/mol |
| Absorption* | λmax (n.a) |
| Fluorescence | λem (n.a.) |
| HOMO/LUMO | HOMO = 5.42 eV, LUMO = 2.3 eV |
| Synonyms | Cl-4BPT, (4-(3,7-Dichloro-10H-phenothiazin-10-yl)butyl)phosphonic acid |
| Classification or Family | 10H-phenothiazine derivatives, Self-assembly Monolayers, Hole transport layer, Hole extraction layer, p-i-n Perovskite solar cells, Organic photovoltaics |
Product Details
| Purity | > 98% (HPLC) |
|---|---|
| Melting Point | N/A |
| Appearance | White powder/crystals |
Chemical Structure
MSDS Documentation
Literature and Reviews
- M. Li et al. (2024); A Hole-Selective Self-Assembled Monolayer for Both Efficient Perovskite and Organic Solar Cells, Langmuir, 40 (9), 4772–4778; DOI: 10.1021/acs.langmuir.3c03610.
- A. Ullah et al. (2022); Novel Phenothiazine-Based Self-Assembled Monolayer as a Hole Selective Contact for Highly Efficient and Stable p-i-n Perovskite Solar Cells, Adv. Energy Mater., 12 (2), 2103175; DOI: 10.1002/aenm.202103175.
- Z. Li et al. (2023); Simple and robust phenoxazine phosphonic acid molecules as self-assembled hole selective contacts for high-performance inverted perovskite solar cells, Nanoscale, 15, 1676-1686; DOI: 10.1039/D2NR05677A.
Licensed by Helmholtz-Zentrum Berlin für Materialien und Energie GmbH in Germany and Kaunas University of Technology in Lithuania.
