Br-2EPT
CAS Number 2826271-17-2
Charge Transport Layer Materials, Materials, OLED Materials, Perovskite Interface Materials,Small Self-Assembled Monolayer Molecule for High Efficiency Solar Cell
Br-2EPT, Hole transport or extraction layer for NFA-polymer solar cells and p-i-n perovskite solar cells, (2-(3,7-dibromo-10H-phenothiazin-10-yl)ethyl)phosphonic acid, CAS No. 2826271-17-2
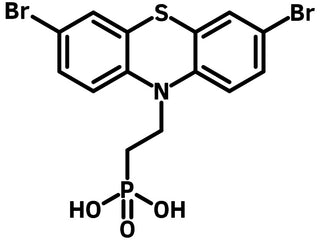
Br-2EPT is a self-assembled monolayer (SAM) material that assembles 3,7-dibromo-10H-phenothiazine as the terminal, ethyl chain as the spacer and phosphonic acid as the anchor to the surface of the electrode.
Br-2EPT self-assembled monolayer enables an interface that is energetically well-aligned with the perovskite active layer materials, which minimizes nonradiative interfacial recombination loss by effectively separating charges on the interface. With reduced HOMO energy level induced by bromide groups to minimize the energy-band shift and energy loss, higher VOC can be achieved. The bromine substituents in Br-2EPT form halogen bonds with perovskite and the sulfur atoms in phenothiazine passivate the Lewis acid defects. As a result, Br-2EPT as hole selective contact, hence significantly improves the charge extraction/transport with enhanced device efficiency and long-term stability.
Serving as hole selective contact for organic solar cells and perovskite solar cells, Br-2EPT is an alternative to PEDOT:PSS with superior performance with the convenience of solution deposition at low concentration, i.e. 1 mM.
Solution Processing Procedure
Typical processing solvents: Anhydrous ethanol (methanol, IPA, IPA/DMF, THF are also superior solvents)
Typical concentration: 1 mM (0.465 mg/mL) - 1.0 mg/ml
Typical processing procedure: 100 uL of Br-2EPT solution (1mM, 0.465 mg in 1 ml ethanol) is deposited onto the center of the UVO treated FTO or ITO substrate surface and spin-coated for 30 s at the speed of 3000 rpm then annealed at 100 ℃ for 10 minutes (DOI: 10.1002/aenm.202103175)
General Information
| CAS Number | 2826271-17-2 |
|---|---|
| Chemical Formula | C14H12Br2NO3PS |
| Molecular Weight | 465.10 g/mol |
| Absorption* | λmax (n.a) |
| Fluorescence | λem (n.a.) |
| HOMO/LUMO | HOMO = 5.47 eV, LUMO = 2.12 eV |
| Synonyms | (2-(3,7-Dibromo-10H-phenothiazin-10-yl)ethyl)phosphonic acid |
| Classification or Family | 10H-phenothiazine derivatives, Self-assembly monolayers, Hole transport layer, Hole extraction layer, p-i-n Perovskite solar cells, Organic photovoltaics |
Product Details
| Purity | > 98% (HPLC) |
|---|---|
| Melting Point | n.a. |
| Appearance | Off-white powder |
Chemical Structure
MSDS Documentation
Literature and Reviews
-
Novel Phenothiazine-Based Self-Assembled Monolayer as a Hole Selective Contact for Highly Efficient and Stable p-i-n Perovskite Solar Cells, A. Ullah et al., Adv. Energy Mater., 12 (2), 2103175 (2022); DOI: 10.1002/aenm.202103175.
-
Versatile Hole Selective Molecules Containing a Series of Heteroatoms as Self-Assembled Monolayers for Efficient p-i-n Perovskite and Organic Solar Cells, A. Ullah et al., Adv. Funct. Mater., 32, 2208793 (2022); DOI: 10.1002/adfm.20220879310.1002/aenm.202103175.
-
Advantages and challenges of self-assembled monolayer as a hole-selective contact for perovskite solar cells, S. Wang et al., Mater. Futures, 2, 012105 (2023); DOI 10.1088/2752-5724/acbb5a.
-
Co-deposition of hole-selective contact and absorber for improving the processability of perovskite solar cells, X. Zheng et al., Nat Energy 8, 462–472 (2023); DOI: 10.1038/s41560-023-01227-6.
-
A Comprehensive Review of Organic Hole-Transporting Materials for Highly Efficient and Stable Inverted Perovskite Solar Cells, Y. Duan et al., 2315604 (2024); DOI: 10.1002/adfm.202315604.
Licensed by Helmholtz-Zentrum Berlin für Materialien und Energie GmbH in Germany and Kaunas University of Technology in Lithuania.
