TAZ, 3-(Biphenyl-4-yl)-5-(4-tert-butylphenyl)-4-phenyl-4H-1,2,4-triazole
TAZ, efficient ETL and HBL in blue PhOLEDs
High-purity (>99.0%) and available online for priority dispatch
Overview | Specifications | Pricing and Options | MSDS | Literature and Reviews
1,2,4-triazole-based 3-(biphenyl-4-yl)-5-(4-tertbutylphenyl)-4-phenyl-4H-1,2,4-triazole (TAZ) (ET: 2.7 eV, HOMO/LUMO: 6.3/2.7 eV) has mostly been used in blue phosphorescent OLEDs (PhOLEDs) to serve as an efficient electron-transporting and hole-blocking layer due to its high triplet energy level that would confine the triplet excitons within the emissive layer.
The low HOMO/LUMO energy level of TAZ is beneficial for blocking holes and facilitating electron injection/transport, thereby enhancing the device performance.
General Information
| CAS Number | 150405-69-9 |
|---|---|
| Chemical Formula | C30H27N3 |
| Molecular Weight | 429.56 g/mol |
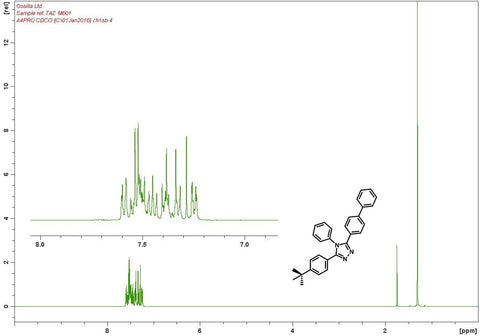
| Absorption | λmax 280 nm in chloroform |
| Fluorescence | λem 372 nm in chloroform |
| HOMO/LUMO | HOMO = 6.3 eV, LUMO = 2.7 eV [1] |
| Synonyms |
TAZ |
| Classification / Family | Triazole derivatives, Electron-injection layer materials (EIL), Electron-transport layer materials (ETL), Hole-blocking layer materials (HBL), Phosphorescent host materials, Organic light-emitting diodes (OLEDs), Organic electronics. |
Product Details
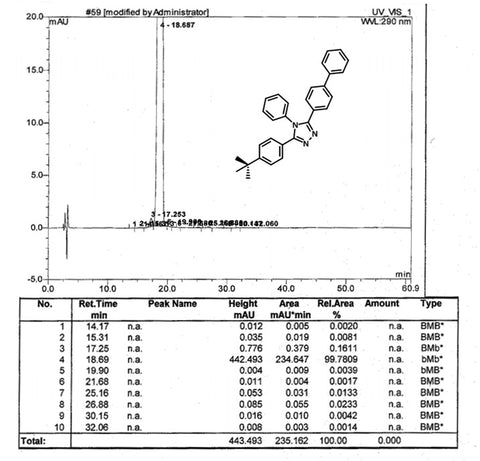
| Purity | >99.0% (sublimed) |
|---|---|
| Melting Point | 231 – 235 °C (lit.) |
| Appearance | White powder/crystals |
*Sublimation is a technique used to obtain ultra pure-grade chemicals. For more details about sublimation, please refer to the Sublimed Materials.
Chemical Structure
Device Structure(s)
| Device Structure | ITO/PEDOT:PSS/α-NPD (20 nm)/TCTA (5 nm)/T2T*:(PPy)2Ir(acac)(9:1 wt%) (25 nm)/TAZ (50 nm)/LiF (0.5 nm)/Al (100 nm) [1] |
|---|---|
| Color | Green |
| Max. Luminance | 85,000 cd/m2 |
| Max. Current Efficiency | 54 cd/A |
| Max. EQE | 17.4% |
| Max. Power Efficiency | 48 lm W−1 |
| Device Structure | ITO/PEDOT:PSS (50 nm)/poly-TCZ (35 nm)/1*:Ir(ppy)3 (94:6 wt%)(20 nm)/TAZ (50 nm)/LiF (2.5 nm)/Al (40 nm)/Ag (100 nm) [2] |
|---|---|
| Color | Blue |
| Max. Luminance | 47,000 cd/m2 |
| Max. Current Efficiency | 81.1 cd/A |
| Max. EQE | 25.2% |
| Max. Power Efficiency | 46.8 lm W−1 |
| Device Structure | MoO3 (3 nm)/CBP: 20 wt% Ir(ppy)3: 4 wt% FIrpic (30 nm)/TAZ (50 nm) [3] |
|---|---|
| Color | Green |
| Max. Luminance | 27,524 cd/m2 |
| Max. Current Efficiency | 71.2 cd/A |
| Device Structure | ITO/NPB (50nm)/mCP (10 nm)/CbzTAZ:15 wt%FIrpic (35 nm)/TAZ (30 nm)/LiF (1 nm)/Al (120 nm) [5] |
|---|---|
| Color | Blue |
| Max. EQE | >23% |
| Max. Current Efficiency | >45 cd/A |
| Max. Power Efficiency | >40 lm W−1 |
| Device Structure | ITO/MoO3 (7nm)/NPB (85 nm)/ (PPQ)2Ir(acac):Ir(ppy)3:FIrpic:mCP/TAZ/LiF/Al [6] |
|---|---|
| Color | White |
| Max. EQE | 20.1% |
| Max. Power Efficiency | 41.3 lm W−1 |
| Device Structure | ITO/HATCN (5 nm)/NPB (40 nm)/TCTA (10 nm)/mCP:6 wt%2CzPN (11 nm)/TAZ:4 wt% PO-01 (4 nm)/TAZ (40 nm)/LiF (0.5 nm)/Al (150 nm) [7] |
|---|---|
| Color | White |
| Max. EQE | 38.4% |
| Max. Power Efficiency | 80.1 lm W−1 |
*For chemical structure information please refer to the cited references.
Characterization
Pricing
| Grade | Order Code | Quantity | Price |
|---|---|---|---|
| Sublimed (>99.0% purity) | M601 | 250 mg | £175 |
| Sublimed (>99.0% purity) | M601 | 500 mg | £290 |
| Sublimed (>99.0% purity) | M601 | 1 g | £460 |
MSDS Documentation
Literature and Reviews
- 1,3,5-Triazine derivatives as new electron transport–type host materials for highly efficient green phosphorescent OLEDs, H-Fan Chen et al., J. Mater. Chem., 19, 8112–8118 (2009).
- Efficient blue-emitting electrophosphorescent organic light-emitting diodes using 2-(3,5-di(carbazol-9-yl)-phenyl)-5-phenyl-1,3,4-oxadiazole as an ambipolar host, Y. Zhang et al., RSC Adv., 3, 23514 (2013). DOI: 10.1039/c3ra43720e.
- Simplified phosphorescent organic light-emitting devices using heavy doping with an Ir complex as an emitter, Y. Miao et al., RSC Adv., 5, 4261 (2015). DOI: 10.1039/c4ra13308k.