n-Butylammonium Bromide
CAS Number 15567-09-6
Materials, Perovskite Materials, Perovskite Precursor Materialsn-Butylammonium bromide, high quality perovskite precursor
Used to tune perovskite nanoplatelet structures for perovskite LEDs and solar cells
MSDS | Literature and Reviews | Resources and Support
| CAS number | 15567-09-6 |
| Chemical formula | C4H12BrN |
| Molecular weight | 154.05 g/mol |
| Synonyms |
|
| Classification / Family | Perovskite precursor materials, Perovskite solar cells, Perovskite LEDs |
Applications
N-Butylammonium bromide (BABr) is commonly used to tune colloidal perovskite nanoplatelet structures for desired absorption/emission energy and device performance for perovskite LEDs and Solar cells. [1]
When larger-group ammonium halides that act as surfactants (such as BAI or BABr) are added to the perovskite precursor solution, they dramatically constrain the growth of 3D perovskite grain. This results in the production of crystallites with dimensions as small as 10 nm, and a film roughness of less than 1 nm [2].
Product Details
| Purity | ≥ 98% |
| Melting point | 205.4 °C |
| Color | White powder/crystals |
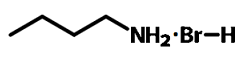
Chemical Structure
| Device structure | ITO (150 nm)/HTL (30 nm)/BABr:MAPbBr3 (20:100) (70 nm) /TPBi (60 nm)/LiF (1.2 nm)/Al (100 nm) [2] |
| Color |
|
| Current Efficiency @ 1000 cd/m2 | 17.1 cd/A |
| MAax Power Efficiency | 13.0 lm W−1 |
| Max EQE | 9.3% |
MSDS Documentation
n-Butylammonium bromide MSDS Sheet
Literature and reviews
- Highly Tunable Colloidal Perovskite Nanoplatelets through Variable Cation, Metal, and Halide Composition, M. C. Weidman et al., ACS Nano, 10 (8), 7830–7839 (2016); DOI: 10.1021/acsnano.6b03496.
- Efficient perovskite light-emitting diodes featuring nanometer-sized crystallites, Z. Xiao et al., Nat. Photonics, 11, 108–115 (2017); doi:10.1038/nphoton.2016.269.
- Efficient ambient-air-stable solar cells with 2D–3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites, Z. Wang et al., Nat. Energy, 6 17135 (2017); doi:10.1038/nenergy.2017.135.
