F8T2
CAS Number 210347-56-1
Chemistry Building Blocks, Interface Polymers, Luminosyn™ Polymers, Materials,F8T2, popular semiconducting polymer used in organic electronics
High purity and available online for priority dispatch
Specifications | MSDS | Literature and Reviews | Technical Support
Poly(9,9-dioctylfluorene-alt-bithiophene), also known as F8T2 (CAS number 210347-56-1), is a semiconducting material that is widely used in organic electronics such as organic photovoltaics, polymer light-emitting diodes (PLED) and organic field-effect transistors (OFETs). Compared with poly-3-hexylthiophene, F8T2 has even higher mobilities of 0.1 cm2/V·s and relatively higher stability against chemical doping by environmental oxygen or residual impurities such as mobile sulphonic acid in PEDOT:PSS ink. This enables devices with higher on-off current ratios exceeding 105 and with better operating stability than printed poly-3-hexylthiophene devices[1].
The absorption in the blue region of F8T2 makes it an excellent donor polymer to blend with an acceptor having complementary spectrum or assemble a tandem cell with other low bandgap-conjugated polymers with absorption extended in the red region.
For 5 - 10 gram order quantities, please enquire, the lead time is 4-6 weeks.
The Luminosyn™ Range
General Information
| CAS Number | 210347-56-1 |
|---|---|
| Chemical Formula | (C37H44S2)n |
| Molecular Weight | See batch information for details |
| HOMO / LUMO | HOMO = 5.5 eV / LUMO = 3.1 eV [1] |
| Synonyms | PFOT, Poly(9,9-dioctylfluorene-alt-bithiophene), Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-bithiophene] |
| Classification / Family | Polyfluorenes, Bithiophenes, Heterocyclic five-membered ring, Organic semiconducting materials, PLED green emitter materials, Organic Photovoltaics, Polymer Solar Cells, Light-emitting Diodes, OFET materials |
| Soluble in | o-Xylene, chloroform, chlorobenzene, dichlorobenzene |
| Recommended Processing Solvents at 10mg/ml | Dichlorobenzene or Chlorobenzene+dichlorobenzene (1:1 v/v) |
Batch Details
| Batch number | MW | Mn | PDI | Stock info |
|---|---|---|---|---|
| M0501A3 | 191,100 | 93,015 | 2.05 | In Stock |
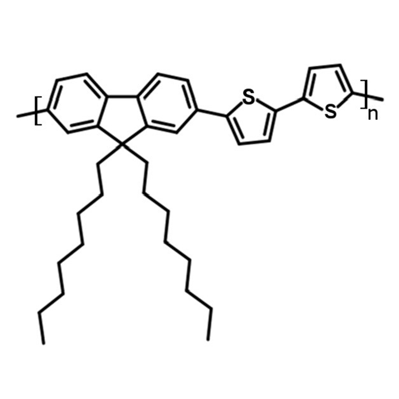
Chemical Structure
Device Structure(s)
| Device structure | ITO/PEDOT:PSS/TFB/F8T2/Ca [3] |
|---|---|
| Color | Green |
| Max. Luminance | 23,400 |
| Max. Current Efficiency | 3.68 cd/A |
| Max. Power Efficiency | 2.9 lm W−1 |
MSDS Documentation
Literature and Reviews
- Annealing effect of polymer bulk heterojunction solar cells based on polyfluorene and fullerene blend, J-H. Huang et al., Org. Electronics, 10, 27–33 (2009), doi:10.1016/j.orgel.2008.09.007.
- High-Efficiency Polymer LEDs with Fast Response Times Fabricated via Selection of Electron-Injecting Conjugated Polyelectrolyte Backbone Structure, M. Suh et al., ACS Appl. Mater. Interfaces, (2015), DOI: 10.1021/acsami.5b07862.
- On the use and influence of electron-blocking interlayers in polymer light-emitting diodes, R. Jin et al., Phys. Chem. Chem. Phys., 11, 3455-3462 (2009). DOI: 10.1039/B819200F.
