Bebq2
CAS Number 148896-39-3
Charge Transport Layer Materials, Electron Transport Layer Materials, High Purity Sublimed Materials, Materials, OLED Materials, Semiconducting MoleculesBebq2, a blue-fluorescence emitter with excellent charge transport ability
Best for use as an ETL material. Available online for priority dispatch
Bis(10-hydroxybenzo[h]quinolinato)beryllium, known as Bebq2, is the brother of Bepp2 in the Beryllium complex family. It is also a blue-fluorescence emitter with excellent charge transport ability.
When compared with Alq3 as an electron-transport material, Bebq2 was proven to be superior, even though the ionization potential and optical band gap of Bebq2 (5.5 eV and 2.7 eV respectively) and Alq3 (5.6 eV and 2.8 eV respectively) are almost the same. The best use for Bebq2 is not as an emitting-layer material or a host material (even though it is a widely used host material), but as an electron-transport material [1].
General Information
| CAS number | 148896-39-3 |
|---|---|
| Chemical formula | C26H16BeN2O2 |
| Molecular weight | 397.43 g/mol |
| Absorption* | λmax 406 nm |
| Fluorescence | λem 440 nm (DCM) |
| HOMO/LUMO | HOMO = 5.5 eV, LUMO = 2.8 eV |
| Synonyms |
|
| Classification / Family | Blue emitter, Electron-transport layer materials (ETL), Hole-blocking layer materials (HBL), Organic light-emitting diode (OLED), Organic electronics |
* Measurable with an optical spectrometer
Product Details
| Purity | >99% (sublimed) |
|---|---|
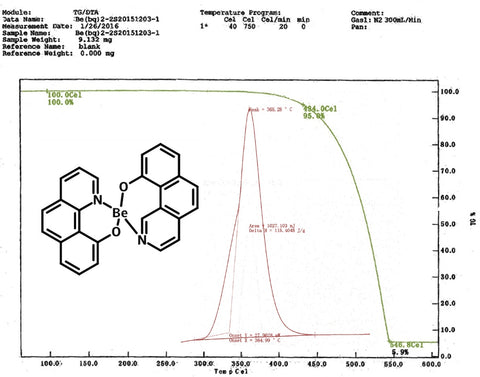
| Thermogravimetric Analysis (TGA) | 434 °C (5% weight loss) |
| Differential Scanning Calorimetry (DSC) | 364.9 °C |
| Color | Yellow powder/crystals |
* Sublimation is a technique used to obtain ultra pure-grade chemicals, see sublimed materials.
Chemical Structure
![Chemical Structure of Bis(10-hydroxybenzo[h]quinolinato)beryllium (Bebq2)](https://www.ossila.com/cdn/shop/files/bebq2-bepq2-chemical-structure.png?v=1718724889)
Device Structure(s)
| Device structure | ITO/DNTPD (40 nm)/Bebq2:Ir(piq)3 (50 nm, 4 wt%)/LiF (0.5 nm)/Al (100 nm) [2] |
|---|---|
| Color | Red |
| Max. Current Efficiency | 9.38 cd/A |
| Max. Power Efficiency | 11.72 lm W−1 |
| Device structure | ITO/NPB (40 nm)/Bebq2:1 wt% Ir(piq)3 (30 nm)/Bebq2 (20 nm)/LiF (0.5 nm)/Al (100 nm) [3] |
|---|---|
| Color | Red |
| Max. Current Efficiency | 12.71 cd/A |
| Max. Power Efficiency | 16.02 lm W−1 |
| Device structure | ITO/MeO-TPD: F4-TCNQ (50 nm, 4 wt%)/NPB (20 nm)/MADN:DSAph (25nm, 7 wt%)/Bebq2 (30 nm)/LiF (1 nm)/Al (200 nm) [4] |
|---|---|
| Color | Blue |
| Max. Luminance | 70,645 cd/m2 |
| Max. Current Efficiency | 12.7 cd/A |
| Max. Power Efficiency | 9.1 lm W−1 |
| Device structure | Ag (100 nm)/ITO (10 nm)/DNTPD (30 nm)/NPB (44 nm)/Bebq2:3 wt% Ir(mphmq)2(acac) (20 nm)/Bphen (31 nm)/Bphen: 5 wt% Li (10 nm)/HATCN (7 nm)/NPB (63 nm)/Bebq2: 3 wt% Ir(mphmq)2(acac) (20 nm)/Bphen (40 nm)/Liq (1 nm)/Mg:Ag (10:1; 18 nm)/NPB (60 nm) [5] |
|---|---|
| Color | Red |
| Max. EQE | 26.5% |
| Max. Current Efficiency | 95.8 cd/A |
| Device structure | ITO/NPB (40 nm))/AND:3 wt% DPAVBi (45 nm)/Bebq2 (15 nm)/LiF (1.2 nm)/Al (100 nm) [6] |
|---|---|
| Color | Blue |
| EQE @ 100 cd/m2 | 8.32% |
| Current Efficiency @100 cd/m2 | 19.2 cd/A |
| Device structure | ITO/PEDOT:PSS (30 nm)/α-NPD (40 nm)/Bebq2:TLEC-025* (1 wt%, 35 nm)/TPBI (40 nm)/LiF (1 nm)/Al (100 nm) [7] |
|---|---|
| Color | Red |
| EQE @ 100 cd/m2 | 17.1% |
| Power Efficiency @100 cd/m2 | 13.6 lm W−1 |
| Device structure | ITO/a-NPB:Bebq2:Ir(piq)3 (1 wt.%, 100 nm)/ LiF (0.5 nm)/Al (100 nm) [8] |
|---|---|
| Color | Red |
| Max. Current Efficiency | 9.44 cd/A |
| Max. Purrent Efficiency | 10.62 lm W−1 |
*For chemical structure information please refer to the cited references
Characterization (TGA and DSC)
MSDS Documentation
Literature and Reviews
- Influence of the Emission Site on the Running Durability of Organic Electroluminescent Devices, Y. Hamada et al., Jpn. J. Appl. Phys., 34, L824 (1995); http://iopscience.iop.org/1347-4065/34/7A/L824.
- Efficiency Control in Iridium Complex-Based Phosphorescent Light-Emitting Diodes, B. Diouf et al., Adv. Mater. Sci.&Eng., 2012, 794674 (2012); doi:10.1155/2012/794674.
- Highly Efficient Simple-Structure Red Phosphorescent OLEDs with an Extremely Low Doping Technology, W. S. Jeon et al., J. Info. Display, 10 (2), 87-91 (2009).
