PBBTSiD
CAS Number 1334032-16-4
Luminosyn™ Polymers, Materials, OPV Polymers, Semiconducting PolymersSpecifications | MSDS | Literature and Reviews | Technical Support
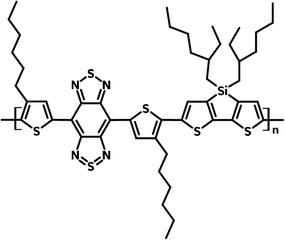
PBBTSiD (CAS number 1334032-16-4) is a small bandgap copolymer (Eg = ~ 0.7 eV ) with a backbone alternating electron donating dithienosilole (DTS) and electron accepting fused-ring benzobisthiadiazole (BBT) units.
The balanced ambipolarity of the BBT moiety of the polymer makes PBBTSiD a good candidate for logic circuit applications. Integrating BBT backboned semiconducting polymers into the bottom gate/top contact TFTs resulted in balanced ambipolar performances with the high µh and µe charge mobility values.
For 5 - 10 grams order quantity, the lead time is 4-6 weeks.
The Luminosyn™ Range
General Information
| Full name | Poly[(4,7-bis(3-hexylthien-2-yl)- 2λ4δ2-benzo[1,2-c;4,5-c′]bis[1,2,5] thiadiazole)-alt-(3,3'-bis(2-ethylhexylsilyene-2,2'-bithiophene)] |
| Synonyms | PBBTSiD |
| Chemical formula | (C50H64N4S 6Si )n |
| CAS number | 1334032-16-4 |
| HOMO / LUMO | HOMO = -4.80 eV, LUMO = -4.10 eV; Eg = ~ 0.7 eV [1] |
| Soluble in | Chloroform, chlorobenzene and dichlorobenzene |
| Recommended Processing Solvents at 10mg/ml | Dichlorobenzene |
| Classification / Family | Organic semiconducting materials, Very low-bandgap polymers, Ambipolar semiconducting polymers, OFET polymers, High charge mobility polymers, Photodetectors, Thin-film Transistors |
Batch Details
| Batch | Mw | Mn | PDI | Stock Info |
| M2258A1 | 17,975 | 7,554 |
2.38 | In stock |
Chemical Structure
UV-Vis-NIR Absorption
MSDS Documentation
Literature and Reviews
- Ambipolarity in Benzobisthiadiazole-Based Donor–Acceptor Conjugated Polymers, J. D. Yuen et al., Adv. Mater., 23, 3780–3785 (2011); DOI: 10.1002/adma.201101134.
- Organic Transistors in the New Decade: Toward n-Channel, Printed, and Stabilized Devices, S. Kola et al., J. Polym. Sci. B: Polym. Phys., 50, 1090–1120 (2012); DOI: 10.1002/polb.23054.
- Benzothiadiazole and its π-extended heteroannulated derivatives: useful acceptor building blocks for high-performance donor–acceptor polymers in organic electronics, Y. Wang et al., J. Mater. Chem. C, 4, 6200 (2016); DOI: 10.1039/c6tc01860b.
