Polycyclic Aromatic Hydrocarbons (PAHs)
Superbenzene, [6]Circulene, Cyclobenzene, Sublimed (≥99%) and unsublimed (≥98%)
Specifications | MSDS | Literature and Reviews | Related Products
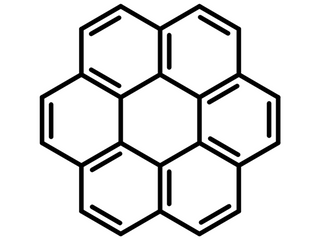
Coronene is a honeycomb shaped symmetric polycyclic aromatic hydrocarbons (PAHs) composed from six peri-fused adjacent benzene rings. It can be visualized as one central benzene fully enclosed by outer fused benzene rings. Coronene is also known as cyclobenzene, superbenzene, or as a nanoflake fragment of graphene. Coronene dissolves in common solvents such as toluene, benzene and dichloromethane. When exposed to UV light, coronene solutions glow blue. The high conjugation of coronene and its high intrinsic carrier mobility make it a great candidate for applications in organic electronics.
Coronene is known as a superaromatic molecule with 24 π-electrons which does not conform to the 4n + 2 Huckel’s aromaticity rule. RGB emission can be realized from three single-crystals that are composed only of coronene. Yellow crystals of three-dimensional (3D) rod-crystal and one-dimensional (1D) wire-crystal exhibit different emission band under UV-light irradiation, blue and green, respectively, while the short and flat two-dimensional (2D) plate-crystal of coronene dimer exhibits red emission band.
CDIN, an amine functionalized discotic coronene diimide with an expanded π-conjugated planar structure, enables efficient face-on π-π stacking in the solid states and charge carrier mobility in vertical direction. Moreover, the coronene based electron transport material offers superior transparency and mobility and shows a relatively thickness insensitive feature for the derived OPVs up to the thickness of 25 nm.
General Information
| CAS Number | 191-07-1 |
|---|---|
| Full Name | Coronene |
| Synonyms | [6]circulene, cyclobenzene |
| Chemical Formula | C24H12 |
| Molecular Weight | 300.36 g/mol |
| Absorption | λab 310.1 nm, 327.2 nm, 342.7 nm (in TCB) |
| Fluorescence | λem 447 nm (in CF) |
| Classification/Family | Polycyclic aromatic hydrocarbons (PAHs), Organic electronics |
Chemical Structure
Product Details
| Purity | Unsublimed >98.0% (1H NMR), Sublimed > 99% |
|---|---|
| Melting Point | 440 °C |
| Appearance | Yellow to Brown to Dark green powder/crystals |
MSDS Documentation
Literature and Reviews
- T. Nakagawa et al. (2025); RGB photoluminescence from single-component hydrocarbon single-crystals: Revealing excited-state dynamics in organic semiconductors, Carbon, 233, 119873; DOI: 10.1016/j.carbon.2024.119873.
- J. Yu et al. (2017); Boosting performance of inverted organic solar cells by using a planar coronene based electron-transporting layer, Nano Energy, 39, 454-460; DOI: 10.1016/j.nanoen.2017.07.031.
- S. Kumar et al. (2021); Coronenes, Benzocoronenes and Beyond: Modern Aspects of Their Syntheses, Properties, and Applications, Chem. Asian J., 16 (6), 621-647; DOI: 10.1002/asia.202001465.