Small Self-Assembled Monolayer Molecule for High Efficiency Solar Cells
Hole transport or extraction layer for NFA-polymer solar cells and p-i-n perovskite solar cells
Product Information | MSDS | Literature and Reviews
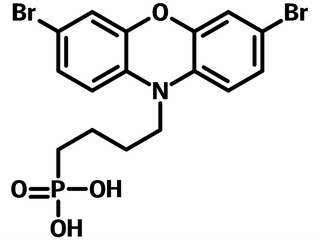
2BrPXZPA is a self-assembled monolayer material bearing an electron donating 3,7-dibromo-10H-phenoxazine as the terminal, a n-butyl alkyl chain, and a phosphonic acid anchor. Structurally, phenoxazine (PXZ) can be considered as one of the typical examples of doping oxygen atoms in carbazole. In perovskite solar cells, where lead (Pb) is one of the active ingredients, the oxygen (O) atom has stronger coordination with Pb than sulfur (S). The bromine atoms from the terminal function group are there to tune the electronic structure band alignment between the self-assembled monolayer material 2BrPXZPA and active perovskites, and naturally coordinate the exposed Pb2+ from perovskite.
Device power conversion efficiency (PCE) of 22.93% was achieved for a perovskite solar cell with the following device structure when 2BrPXZPA was used as the hole selective contact. The fabricated device also showed promising long-term stability with 97% of the initial efficiency retained after 600 hours of light soaking under one standard sun illumination (AM 1.5G). Moreover, superior PCE of 23.66% was obtained with 2BrPXZPA modified NiOx hole selective layer, improved from 20.55% of the control NiOx-based device.
Device structure: ITO/2BrPXZPA/Cs0.05(FA0.85MA0.15)0.95Pb(I0.85 Br0.15)3 /PC61BM/BCP/Ag
Serving as hole selective contact for organic solar cells and perovskite solar cells, 2BrPXZPA is an alternative to PEDOT:PSS with superior performance and the convenience of solution deposition at low concentration, i.e. 1 mM.
Solution Processing Procedure
Typical processing solvents: THF, ethanol, methanol, IPA, DMF
Typical concentration: 1 mM (0.477 mg/ml) or 1.0 mg/ml (1 mg 2BrPXZPA dissolved in 1 ml THF)
Typical processing procedure: 30 uL of 2BrPXZPA in THF solution (1.0 mg/ml) is deposited onto the center of the substrate surface and spin-coated for 30 s at the speed of 3000 rpm. The coated film on the substrate is then annealed for 10 min at 100 °C (DOI: 10.1039/D2NR05677A).
General Information
| CAS Number | N/A |
|---|---|
| Chemical Formula | C16H16Br2NO4P |
| Molecular Weight | 477.08 g/mol |
| Absorption* | λmax (334 nm in THF, 342 nm in film) |
| Fluorescence | λem (n.a.) |
| HOMO/LUMO | HOMO = 5.46 eV, LUMO = 2.43 eV |
| Synonyms | Br-4BPO, (4-(3,7-dibromo-10H-phenoxazin-10-yl)butyl)phosphonic acid |
| Classification or Family | 10H-phenoxazine derivatives, Self-assembly monolayers, Hole transport layer, Hole extraction layer, p-i-n Perovskite solar cells, Organic photovoltaics |
Product Details
| Purity | > 98% (HPLC) |
|---|---|
| Melting Point | Td = 225 °C |
| Appearance | Blue powder/crystals |
Chemical Structure
MSDS Documentation
Literature and Reviews
- Z. Li et al. (2023); Simple and robust phenoxazine phosphonic acid molecules as self-assembled hole selective contacts for high-performance inverted perovskite solar cells, Nanoscale, 15, 1676-1686; DOI: 10.1039/D2NR05677A.
- Z. Yi et al. (2023); Self‐assembled monolayers (SAMs) in inverted perovskite solar cells and their tandem photovoltaics application, Interdisciplinary Mater., 3, 203–244; DOI: 10.1002/idm2.12145.
- W. Li et al. (2024); Self-assembled molecules as selective contacts for efficient and stable perovskite solar cells, Mater. Chem. Front., 8, 681-699; DOI: 10.1039/d3qm01017a.
Licensed by Helmholtz-Zentrum Berlin für Materialien und Energie GmbH in Germany and Kaunas University of Technology in Lithuania.
