What are Fullerenes?
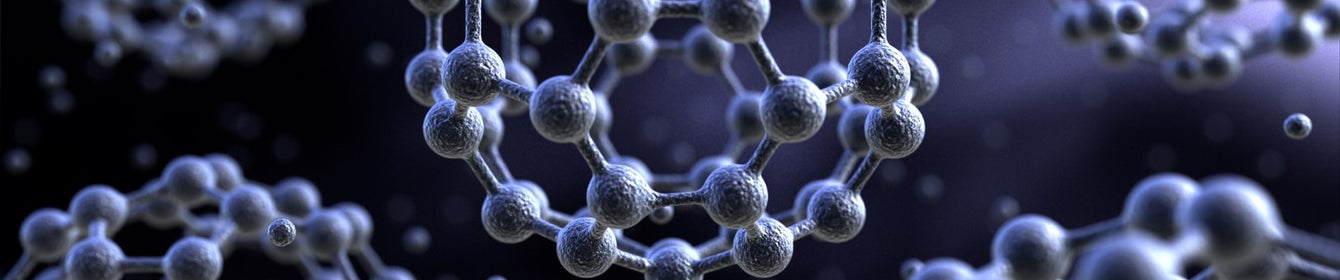
Fullerenes are an allotrope of carbon and are known for their hollow, cage-like structures. Examples include fullerene C60 (also known as carbon-60) and its closely related compound PCBM. Their structures a made from carbon atoms bonded together to form hexagonal and pentagonal rings, like the pattern found on a soccer ball/football or geodesic dome.
The First Fullerene Discovered
The first to be discovered and most famous fullerene, C60, is known as buckminsterfullerene or "buckyball". It was discover in September 1985 at Rice University, Huston, Texas. The name was inspired by the architect Buckminster Fuller, who designed geodesic domes with similar structures.
Fullerene C60 was named "Molecule of the Year" by the journal Science in 1991. This recognition was due to its unique structure and potential implications in various scientific fields.
"When the robots of nanotechnology start playing soccer-football, they will have the perfect molecule to kick around, a buckyball"
Who Discovered Fullerene?
Sir Harold W. Kroto, Robert F. Curl, Jr., and Richard Smalley were awarded with the Nobel Prize in Chemistry in 1996 for the discovery of C60 fullerenes. They recreated the conditions of a star in order to access this new form of carbon molecule. By shining a laser at a graphite disk, carbon atoms become vaporized creating a plume. When this cools carbon bonds form which result in the formation of fullerene. This led to the exploration of other fullerene structures and their potential applications.
Structure of Fullerene
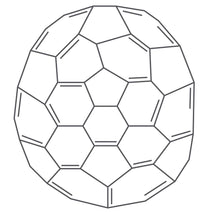
The key structural characteristics of fullerenes include:
Closed-Cage Structure: Fullerenes are closed-cage molecules where carbon atoms form a continuous surface with no dangling bonds. This structure contributes to their stability and resilience.
Pentagons and Hexagons: The arrangement of carbon atoms in fullerenes includes a combination of pentagonal and hexagonal rings. In the case of C60, the molecule consists of 12 pentagons and 20 hexagons.
Symmetry: Fullerenes, especially C60, exhibit a high degree of symmetry, making them aesthetically pleasing and scientifically intriguing. The symmetrical arrangement of carbon atoms contributes to the unique electronic and physical properties of fullerenes.
Variability in Size: Fullerenes can vary in size, with the number of carbon atoms ranging from less than 60 to more than 100. The properties of fullerenes can change significantly with their size and structure. In theory, an infinite number of fullerenes can exist, their structure based on pentagonal and hexagonal rings, constructed according to rules for making icosahedra.
What Type of Solid is Fullerene?
C60 fullerene belongs to the class of Archimedean solids. This is a class of solid materials where all their faces are regular polygons (eg. hexagons and pentagons) and all the vertices (where at least two faces meet) are symmetric to each other.
In C60 there are twenty hexagons and 12 pentagons. No two pentagons share an edge and so fullerene C60 is described as having isolated pentagons. The edges of each pentagon join only hexagons, and the edges of each hexagon alternately join pentagons and hexagons.
Fullerene Bonding
Each carbon atom in C60 forms two single bonds with the sides of a pentagon and one double bond with the edge of two neighboring hexagons. The bond angles within fullerene are therefore 120° and 108° (the standard bond angles for hexagons and pentagons respectively). For different fullerenes with more carbons or different chemical functional groups the bond angles change. This is because in order to accommodate the new atoms some of the faces become irregular polygons and so the bond angles change.
The curved shape of the C60 molecule is caused by the strain from the pentagons. This changes the usual flat bonding for benzene-like structures, thereby limiting the flow of electrons through the molecule.
Fullerenes do have some delocalization of electrons though due to the double bonds forming in the hexagons. Therefore, there are some π-electrons which contribute to the unique electronic properties of fullerenes, such as their ability to act as electron acceptors and their varied reactivity.
Examples of Fullerenes
C60 is not the only form of fullerene. There are in fact several types of fullerenes that can be constructed with hexagons and pentagons, each with distinct structures and properties. Some of the most common types include:
Buckminsterfullerene (C60): The most well-known and studied fullerene, consisting of 60 carbon atoms arranged in a truncated icosahedron. It is often referred to as a "buckyball."
Higher Fullerenes: Fullerenes with more than 60 carbon atoms, such as C70, C76, C78, and C84. These molecules have similar cage-like structures but differ in the arrangement and number of pentagons and hexagons.
Heterofullerenes: Fullerenes in which some of the carbon atoms are replaced by other elements, such as nitrogen or boron. These substitutions can modify the electronic and chemical properties of the fullerenes.
Endohedral Fullerenes: Endohedral fullerenes are a special class of fullerenes in which other atoms, ions, or clusters are trapped inside the carbon cage. These encapsulated species can significantly alter the physical and chemical properties of the fullerene, leading to potential applications in fields such as medicine, electronics, and catalysis. An example is the encapsulation of a metal atom inside a fullerene, resulting in metallofullerenes.
Properties of Fullerenes
Fullerenes are known for their exceptional stability and resilience. The cage-like structure provides high strength and resistance to deformation. This means they can withstand high pressures and temperatures without collapsing or decomposing.
The presence of delocalized π-electrons gives fullerenes interesting chemical reactivity, enabling them to participate in various reactions. This include addition, oxidation, and reduction reactions. They can also form stable complexes with metals and other atoms. Fullerenes exhibit unique electronic properties, including semiconducting behavior and the ability to act as electron acceptors. These distinctive properties have led to the exploration and utilization of fullerenes in fields like materials science, electronics, medicine, and environmental science.
Allotropes of Carbon
Fullerenes possess unique structural properties that distinguish them from other carbon allotropes like graphite, diamond, and graphene. Graphite is one of only two naturally occurring forms of pure carbon and it is the most stable form of carbon. Graphite occurs in a two-dimensional, planar molecular structure where multiple layers of graphene held together by weak Van der Waal force, whereas diamonds have a three-dimensional crystal structure. In diamond every carbon atom is strongly linked to four other carbon atoms by strong directional single covalent bonds giving a three dimensional (3D) strong lattice and a very rigid strong structure. Graphene, derived from graphite is the single layer of carbon atoms arranged in a honeycomb nanostructure. If graphite is a book, then graphene is every page.
PCBM
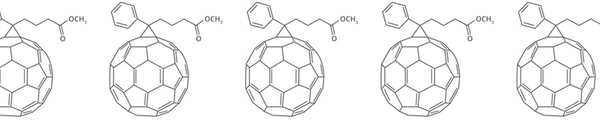
Learn More
 Properties of Fullerene
Properties of Fullerene
Fullerene C6o exhibits unique structural, chemical, electronic, and thermal properties. This is due to its highly symmetrical icosahedral structure that contains a mixture of single and double bonds. All fullerenes have partial electron sharing across the molecule and a high affinity for electrons.
Read more...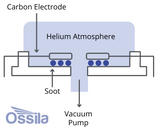 How are Fullerenes Made?
How are Fullerenes Made?
Today, fullerenes are made using three main methods; Huffman-Krätschmer, combustion and microwave. Chemical synthesis techniques, such as laser irradiation and pyrolysis, offer exciting possibilities for producing fullerene derivatives that were previously not accessible.
Read more..References
- Molecule of the Year, Koshland, D. E., Science (1991)
- A geometric principle may guide self-assembly of fullerene..., Schein, S. et al., Biophys J. (2008)
- Beyond C60: the higher fullerenes, Diederich, F. et al., Accounts of Chemical Research (1992)
- An extended cluster expansion for ground states of..., Cheng, Y-H. et al., Scientific Reports (2017)
- Synthesis of endohedral fullerenes by molecular surgery, Bloodworth, S. et al., Communications Chemistry (2022)




