What is an LFP Battery?

LFP is used as an abbreviation for the cathode active material lithium iron phosphate (LiFePO4) powder. It is also known as lithium ferro phosphate which gets shortened to LFP, hence the name. An LFP battery is a type of lithium-ion battery where lithium iron phosphate is used as the cathode material and lithium source. The anode is typically made from a graphite carbon-based material coated on copper foil.
LFP Crystal Structure
Lithium iron phosphate (LFP) is an inorganic crystal belonging to the olivine family of lithium ortho-phosphates. It crystallizes in olivine‐type structure with orthorhombic symmetry, space group Pnma, characterized by a distorted hexagonal anion close packing. This results in an interconnected network composed of phosphate (PO4), iron oxide (FeO6), and lithium oxide (LiO6) units arranged in an olivine-type, orthorhombic crystal lattice.
Phosphate Structures
- Phosphate Tetrahedra: Each phosphate group forms a tetrahedron where a phosphorus atom is covalently bonded to four oxygen atoms.
Lithium Coordination
Lithium atoms bond with six oxygen atoms to form LiO6 octahedra
- Share oxygen atoms with four corner-sharing FeO6 octahedra and two PO4 tetrahedra.
- Share edges with two adjacent LiO6 octahedra, forming chains along the [001] crystallographic direction (b-axis).
- Share edges with two FeO6 and edges with two equivalent PO4 tetrahedra.
Iron Coordination
Similarly, Fe2+ is bonded to six O2- atoms to form FeO6 octahedron:
- Shares corners with four FeO6 octahedra, forming zigzag layers in the (b, c) plane.
- These zigzag layers are stacked along the a-axis through the sharing one edge and two corners of PO4.
This detailed sharing of oxygen atoms among the different polyhedra creates the robust and interconnected lattice characteristic of LFP.
LFP Chemistry
The different chemical components in lithium iron phosphate combine to give a material with properties suited to application as cathode material.
Lithium ions: Li+ are in a stable +1 oxidation state and are relatively small, which makes them highly mobile within the crystal lattice. This mobility allows them to be easily de-intercalated (extracted) and re-intercalated during battery operation without significantly disturbing the lattice.
Iron: Fe acts as a redox center. It changes its oxidation state from +2 to +3 when lithium is extracted to help maintain charge balance. Despite this redox activity, iron ions remain fixed in the lattice to preserve the structural integrity of LFP.
Structural Stability: The rigid tetrahedral shape provides a strong framework that resists distortion, contributing significantly to the mechanical integrity of the lattice.
Electrochemical Impact: The robust phosphate framework is crucial for the electrochemical performance of LFP, as it facilitates ion transport and maintains structural stability during battery operation.
Advantages of LFP Batteries
LFP batteries are a type of lithium-ion battery, and their performance is often evaluated based on key properties. The advantages of lithium iron phosphate (LiFePO4) powder over other lithium-ion chemistries include:
Great Specific Power – the maximum power output per unit of mass is 90 – 170 Wh/Kg
Relatively Cheap – Doesn’t contain rare and expensive metals like other lithium-ion cathode materials.
Great Safety – Phosphate is non-toxic compared to other metal oxides found in cathode materials such as cobalt oxide or manganese oxide.
Great Lifespan - High charge cycle (>10,000 cycles)
Good Performance – Can deliver a constant voltage (~3.2 V) at a high charge cycle (~1500 cycles).
Great Stability - Thermal and chemical stability due to strong P-O bonds.
LFP Battery Structure
An LFP battery consists of an aluminum foil coated with lithium iron phosphate, which serves as the positive cathode. The negative anode is a copper foil coated with graphite. A polymer separator is placed between the electrodes. An electrolyte facilitates the flow of lithium ions between the cathode and anode.
For LFP batteries, during the charging process, lithium ions de-intercalate from LiFePO4. The lithium ions leave the crystal and move toward the anode through the electrolyte. In order to keep a neutral charge balance, electrons also depart from LiFePO4 crystal as the Li-ions. The discharge step sees the lithium ions return to the LFP crystal.
LFP Challenges
While LFP is valued for its stability in battery applications, it also presents several challenges that impact its overall performance and durability. The issues range from limited conductivity and ion diffusion to sensitivity during processing and operation. The following points outline these key challenges.
- LFP suffers from poor electronic conductivity and sluggish Li+ diffusion in the [010] direction.
- Defects in large crystals of LFP blocks the channels for Li to flow out therefore nanoparticles of LFP are better.
- LFP has low electronic conductivity therefore a carbon coating is often used to enhance conductivity.
- LFP electrochemical performance at high or low temperature and high voltage is not ideal.
- LFP nanoparticles are sensitive to air which can undergo significant surface oxidation resulting in a low Coulombic efficiency and poor cycle life.
Cathode Active Materials

Learn More
 What is an LTO Battery?
What is an LTO Battery?
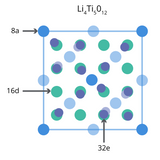
An LTO battery is named after its key component, lithium titanium oxide (LTO) powder. The material is also referred to as lithium titanate with the chemical formula Li4Ti5O12. Unlike most lithium-ion battery materials it is used as the anode active material.
Learn more...Lithium-ion (Li-ion) batteries can catch fire due to a process known as thermal runaway, which is triggered by various factors and involves a series of heat-releasing reactions. While Li-ion batteries are widely used in laptops, cameras, and electric vehicles (EVs) such as scooters and cars, their rise in popularity has not been without issues. In the UK alone, fire services responded to 921 lithium-ion battery fires in 2023, a 46% increase from the previous year.
Learn more...
References
- The origin of fast‐charging lithium iron phosphate for..., Hadouchi, M. et al., battery energy (2021)
Further Reading
- Ahsan, Z., et al. (2020). Recent Progress in Capacity Enhancement of LiFePO4 Cathode for Li-Ion Batteries, ASME. J. Electrochem. En. Conv. Stor. https://doi.org/10.1115/1.4047222
- Chen, R., et al. (2016). Advanced cathode materials for lithium-ion batteries using nanoarchitectonics, Nanoscale Horiz. https://doi.org/10.1039/C6NH00016A
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer