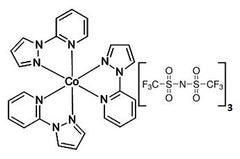
FK102-Co(III)TFSI Salt
CAS Number 2057441-94-6
DSSC Dyes & Electrolytes, Materials, Perovskite Interface Materials, Perovskite MaterialsA pyrazol-pyridine cobalt complex
as an electrolyte for DSSCs and a p-type dopant in perovskite solar cells
Overview | Specifications | MSDS | Literature and Reviews
Tris(2-(1H-pyrazol-1-yl)pyridine)cobalt(III) tri[bis(trifluoromethane)sulfonimide, FK102-Co(III)TFSI Salt is commonly used as electrolyte for DSSC or p-type dopant for Spiro-MeOTAD in perovskite solar cells, together with Lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and 4-tert-Butylpyridine (TBP).
In comparison to triiodide-based redox electrolytes, cobalt complexes in general increase photovoltages and particularly at lower light levels (e.g. for indoor applications), significantly increase device power output.
Cobalt pyrazol-pyridine complex
A p-type dopant for spiro-OMeTAD in perovskite solar cells
Worldwide shipping
Quick and reliable shipping
Large TFSI- anions
stablize the oxidized spiro-OMeTAD
High purity
>98%
General Information
| Full name | Tris(2-(1H-pyrazol-1-yl)pyridine)cobalt(III) tri[bis(trifluoromethane)sulfonimide |
| Synonyms | FK102 |
| Chemical formula | C30H21CoN12O12S6F18 |
| Molecular weight | 1334.87 g/mol |
| CAS number | 2057441-94-6 |
| HOMO / LUMO | n/a |
| Solubility | Acetonitrile |
| Classification / Family | Cobalt complex, Hole transport and dopant materials, Dye Sensitized Solar Cell (DSSC) Materials, Hole Conductor Cobalt Dopants, Organic Photovoltaic (OPV) Materials, Organic and Printed Electronics, Perovskite Materials |
Product Details
| Purity | >98% |
| Melting point | 194-199 °C |
| Appearance | Orange powder |
MSDS Documentation
FK102-Co(III)TFSI Salt MSDS Sheet
Literature and Reviews
- Transformation of the Excited State and Photovoltaic Efficiency of Ch2Nh2PbI3 Perovskite upon Controlled Exposure to Humidified Air, J. A. Christians et al., J. Am. Chem. Soc. 2015, 137, 1530−1538 (2015); DOI: 10.1021/ja511132a.
- Doping and alloying for improved perovskite solar cells, Y. Zhou et al., J. Mater. Chem. A, 4, 17623-17635 (2016); DOI: 10.1039/C6TA08699C.
- Credible evidence for the passivation effect of remnant PbI2 in Ch2Nh2PbI3 films in improving the performance of perovskite solar cells, S. Wang et al., Nanoscale, 8, 6600-6608 (2016); DOI: 10.1039/C5NR08344C.
