LiTFSI
CAS Number 90076-65-6
Battery Materials, Materials, Perovskite Interface Materials, Perovskite MaterialsLiTFSI, high purity p-dopant and battery electrolyte
Used to enhance conductivity and hole mobility of Spiro-OMeTAD for PSCs
Overview | Specifications | Literature and Reviews | Technical Support
Lithium bis(trifluoromethylsulphonyl)imide (LiTFSI), CAS number 90076-65-6, is a hydrophilic salt that can increase the conductivity of a range of organic semiconductors. It is commonly used as a p-dopant to enhance the conductivity and hole mobility of the Spiro-OMeTAD for perovskite solar cells. It is believed to function in PSCs is similar to that in solid-state dye-sensitized solar cells [2].
Some of the lithium ions can intercalate into TiO2 to downshift its conduction band, resulting in a higher photocurrent. The rest of the lithium ions can react with oxygen and Spiro-OMeTAD to facilitate the generation of oxidized Spiro-OMeTAD, while the large anion TFSI¯, can stabilize the oxidized Spiro-OMeTAD as the counterion [1, 2].
LiTFSI has been extensively studied as an electrolyte component in lithium-ion batteries (LIBs) and more. With a high solubility in water (21 molal), concentrated LiTFSI electrolytes can increase the stability of LIBs by reducing the number of water molecules surrounding salt ions. As a result, the water activity responsible for decomposition is decreased.
General Information
| CAS number | 90076-65-6 |
| Chemical formula | C2F6LiNO4S2 |
| Molecular weight | 287.09 g/mol |
| Synonyms |
|
| Classification / Family | Dye Sensitized Solar Cells (DSSC) , Light-emitting Diodes, Perovskite HTL Materials, Electrolyte materials. |
| Storage | Product is hygroscopic. Store under inert atmosphere or in a dessicator. |
Product Details
| Purity | 99.99% |
| Boiling point | 234-238 °C (lit.) |
| Color | White powder/crystals |
Chemical Structure
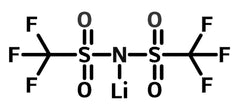
| Device structure | Jsc (mA cm-2) | Voc (V) | FF (%) | PCE (best) |
|---|---|---|---|---|
| FTO/c-TiO2/mp-Al2O3/CH3NH3PbBr3−xClx/CBP/Au [3] | 1.3 | 1.4 | 24 | 0.44 |
| FTO/c-TiO2/mp-Al2O3/CH3NH3PbBr3−xClx/ CBP:(TBP:LiTFSI, 10% wt)/Au | 4 | 1.5 | 46 | 2.7 |
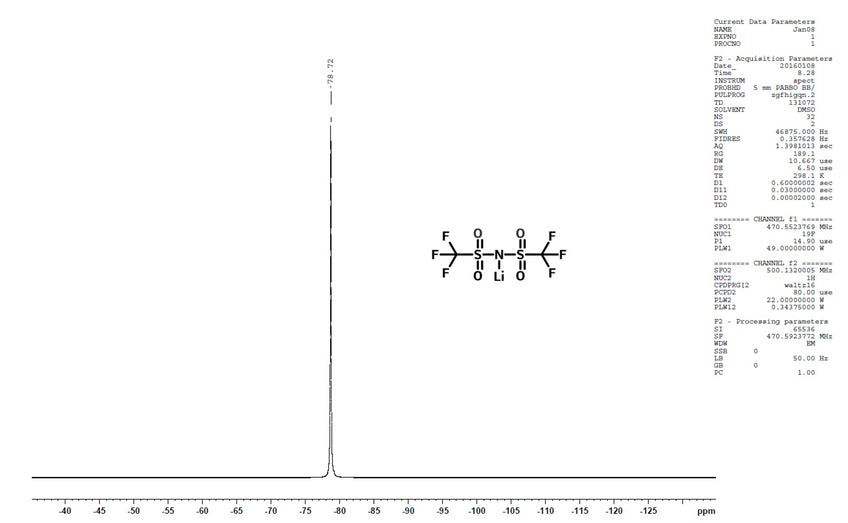
Characterization (NMR)
Literature and Reviews
- Spectrum-Dependent Spiro-OMeTAD Oxidation Mechanism in Perovskite Solar Cells, S Wang et al., ACS Appl. Mater. Interfaces 7, 24791-24798 (2015). DOI: 10.1021/acsami.5b07703.
- Lithium salts as “redox active” p-type dopants for organic semiconductors and their impact in solid-state dye-sensitized solar cells, A. Abate et al., Phys. Chem. Chem. Phys., 15, 2572-2579 (2013). DOI: 10.1039/C2CP44397J.
- Chloride Inclusion and Hole Transport Material Doping to Improve Methyl Ammonium Lead Bromide Perovskite-Based High Open-Circuit Voltage Solar Cells, E. Edri et al., J. Phys. Chem. Lett., 5 (3), 429–433 (2014), DOI: 10.1021/jz402706q.
