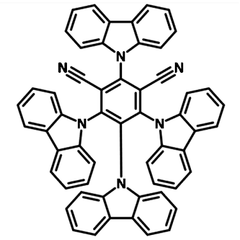
4CzIPN
CAS Number 1416881-52-1
Dopant Materials, Green Dopant Materials, High Purity Sublimed Materials, Materials, OLED Materials, Semiconducting Molecules,4CzIPN, highest PLQY compared to its isomers
Available online in sensible quantities for priority dispatch
Product Information | MSDS | Literature
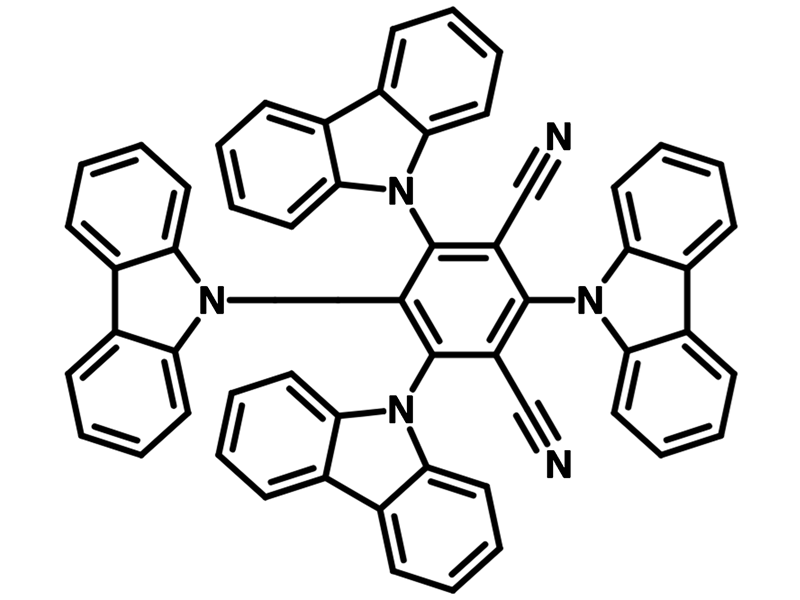
4CzIPN (CAS number 1416881-52-1), namely 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene, has a fully substituted benzene ring with two cyano groups as electron accepting units at meta-positions to each other and four carbazolyl groups as electron donating units. It is a powerful metal-free organophotocatalyst and also a typical donor–acceptor fluorophore.
4CzIPN from Ossila was used in the high-impact paper (IF 15.72), A comprehensive picture of roughness evolution in organic crystalline growth: the role of molecular aspect ratio, J. Dull et al., Mater. Horiz., 9, 2752 (2022); DOI: 10.1039/d2mh00854h.
Out of its three isomers, 4CzIPN has the highest photoluminescence quantum yield (PLQY) of above 90%. This is due to the wide dispersion the highest-occupied molecular orbital (HOMO) over the donor moieties. Relatively short excited-state lifetime of delayed emission was reported. Additionally, higher external quantum efficiency (EQE) was observed by using 4CzIPN as an emitter in TADF-OLED devices.
Despite its low solubility in most of the aromatic solvents, 4CzIPN is also solution-processable in solvents such as dichloromethane or chloroform. This is due to the structure distortion of the carbazole units caused by steric hindrance.
General Information
| CAS Number | 1416881-52-1 |
|---|---|
| Full Name | 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene |
| Chemical Formula | C56H32N6 |
| Molecular Weight | 788.89 g/mol |
| Absorption* | λmax 365 nm in acetonitrile |
| Fluorescence | λem 551 nm in acetonitrile |
| HOMO/LUMO | HOMO = 5.8 eV, LUMO = 3.4 eV [1] |
| Synonyms | 2,4,5,6-Tetra(9H-carbazol-9-yl)isophthalonitrile |
| Classification / Family | Carbazole, TADF green emitter materials, Phosphorescent organic light-emitting devices (PHOLEDs), Photocatalyst, Sublimed materials |
* Measurable with the Ossila USB Spectrometer
Product Details
| Purity | Unsublimed >98% (1H NMR); Sublimed >99.0% (HPLC) |
|---|---|
| Melting Point | TGA: >300 °C (0.5% weight loss) |
| Appearance | Orange-yellow powder/crystals |
Sublimation is a technique used to obtain ultra pure-grade chemicals, see sublimed materials.
Chemical Structure
Device Structure(s)
| Device structure | ITO (70 nm)/(4 wt% ReO 3 ):mCP (50 nm)/mCP (15 nm)/mCP:B3PyMPM:(5 wt% 4CzIPN) (30 nm)/B3PYMPM (20 nm)/(4 wt% Rb2CO3):B3PYMPM (35 nm)/Al (100 nm) [3] |
|---|---|
| Color |
|
| Max. Current Efficiency | 94.5 cd/A |
| Max. EQE | 29.6% |
| Max. Power Efficiency | 88.6 Im/W |
| Device structure | ITO (50 nm)/PEDOT:PSS (60 nm)/poly(9-vinylcarbazole) (15 nm)/SiCz:4CzIPN (30 nm)/TSPO1 (35 nm)/LiF (1 nm)/Al (200 nm) [4] |
|---|---|
| Color |
|
| Max. EQE | 26% |
| Max. Power Efficiency | 63.4 Im/W |
| Device structure | ITO(130 nm)/TAPC (35 nm)/CBP (5 nm)/5 wt% 4CzIPN doped CBP (5 nm)/B4PyPPM (65 nm)/LiF (0.8 nm)/Al (100 nm) [5] |
|---|---|
| Color |
|
| Max. Current Efficiency | 83.2 cd/A |
| Max. EQE | 25.7% |
| Max. Power Efficiency | 106.9 Im/W |
MSDS Documentation
Pricing Table
| Grade | Order Code | Quantity | Price |
|---|---|---|---|
| Sublimed (>99.0% purity) | M2100A1 | 100 mg | £240 |
| Sublimed (>99.0% purity) | M2100A1 | 250 mg | £480 |
| Sublimed (>99.0% purity) | M2100A1 | 500 mg | £760 |
| Sublimed (>99.0% purity) | M2100A1 | 1 g | £1200 |
| Unsublimed (>98.0% purity) | M2100B1 | 250 mg | £230 |
| Unsublimed (>98.0% purity) | M2100B1 | 500 mg | £370 |
| Unsublimed (>98.0% purity) | M2100B1 | 1 g | £580 |
Literature and Reviews
- A comprehensive picture of roughness evolution in organic crystalline growth: the role of molecular aspect ratio, J. Dull et al., Mater. Horiz., 9, 2752 (2022); DOI: 10.1039/d2mh00854h.
- Recent advances of 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) in photocatalytic transformations, T. Shang et al., Chem. Commun., 55, 5408-5419 (2019); DOI: 10.1039/C9CC01047E.
- Promising operational stability of high-efficiency organic light-emitting diodes based on thermally activated delayed fluorescence, H. Nakanotani et al., Sci Rep., 3: 2127 (2013); doi: 10.1038/srep02127.