Carbon Nanotubes vs. Graphene: Structure, Properties, and Uses

Carbon nanotubes, such as single-walled carbon nanotubes (SWCNTs) and graphene are two ground-breaking nanomaterials composed entirely of carbon atoms. Both are allotropes of carbon where atoms are bonded in a hexagonal lattice. Graphene is sheet-like and CNTs are thought of as graphene layers rolled up into a cylinder. Both materials exhibit exceptional electrical, mechanical, and thermal properties. However, their unique structures result in distinct characteristics, making each material better suited for specific applications. The table below highlights some of their key features in comparison.
| Feature | Carbon Nanotubes | Graphene |
|---|---|---|
| Structure |
  |
 |
| Dimensionality | 1D | 2D |
| Electrical Conductivity |
105-106 S m−1 Ranges from metallic to semiconducting depending on structure |
106 S m−1 |
| Young’s Modulus | ~ 1 - 1.28 TPa | 1.1 TPa |
| Tensile Strength | ~ 100 GPa | 130 GPa |
| Transparency | Opaque | Transparent |
| Applications |
|
|
| Cost | More expensive | Cheaper |
CNTs vs Graphene: Structure
Carbon Nanotubes (CNTs)
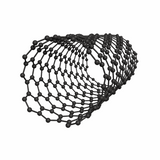
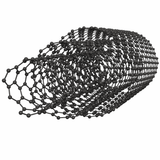
Carbon nanotubes (CNTs) are cylindrical tubes made of rolled-up graphene sheets. They are described as having a tubular structure with a hollow middle. CNTs can exist in different forms but the main two categories are single-walled (SWCNTs) or multi-walled (MWCNTs).
CNTs typically have a diameter of 1–3 nm and their lengths can be several micrometers. As a result they have a 1D (one-dimensional) structure.
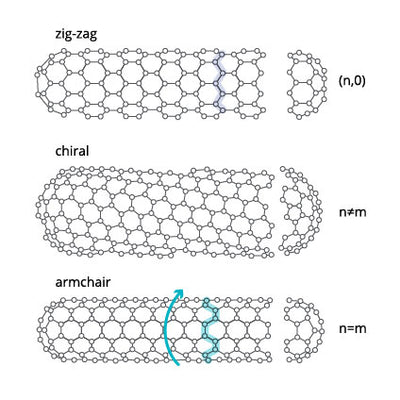
The way in which the graphene sheet is rolled into a cylinder will impact the CNTs orientation or “chirality”. Different chirality gives CNTs with different properties. The different orientations of SWCNT are classified as:
- zig-zag
- armchair
- chiral
Graphene
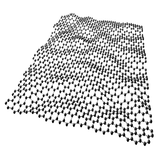
Graphene exists as a single layer of carbon atoms arranged in a hexagonal lattice. It is classed as a 2D (two-dimensional) material and is one carbon atom thick (~0.34 nm).
CNTs vs Graphene: Electrical Properties
Carbon Nanotubes (CNTs)
The electrical properties of carbon nanotubes are heavily reliant on chirality. This controls whether CNTs are semiconducting or metallic. In other words, whether they have a bandgap or not. This means through controlled synthesis nanotubes with specific and desirable electronic properties can be accessed. Metallic CNTs can carry high electrical currents with lower resistance.
Graphene
Graphene has exceptional electron conductivity. This is because it has a zero bandgap and high electron mobility. The bandgap can not be tuned in the same way as CNTs therefore graphene has limited application as a semiconductor. Chemical modifications are used to introduced a bandgap and this can lead to a loss of other excellent properties.
CNTs vs Graphene: Mechanical Properties
Carbon Nanotubes (CNTs)
Carbon nanotubes are extremely strong and light weight, with high tensile strength similar to that of graphene. CNTs have high elasticity and can bounce back from deformation to their original shape.
Graphene
Graphene is the strongest materials known to exist. It is stronger than CNTs with a larger tensile strength. Both materials are used as mechanical reinforcement.
CNTs vs Graphene: Thermal Properties
Carbon Nanotubes (CNTs)
Carbon nanotubes have high thermal conductivity, useful for heat dissipation in electronic devices and thermal management.
Graphene
Graphene is one of the best thermal conductors to exist, with better conductive properties than CNTs. This property makes it an ideal material for increasing heat dissipation within a composite or device. This is helpful for avoiding issues like thermal runaway.
CNTs vs Graphene: Applications
Compared to other nanomaterials CNTs and graphene have similar properties. They are both highly conductive, strong and flexible. This means whilst there are slight differences in the values of their properties they are suited to quite similar applications. Please see the list below.
Carbon Nanotubes (CNTs)
- Electronics: Semiconducting CNTs for transistors and sensors; metallic CNTs for interconnects and circuits.
- Composites: Adding CNTs to materials can enhance strength and electrical conductivity.
- Energy Storage: Used in supercapacitors and batteries due to their large surface area and conductive properties.
Graphene
-
Electronics: Transparent electrodes, transistors, and flexible screens due to its high conductivity and transparency.
- Energy Storage: Similar to CNTs, graphene is used in batteries and supercapacitors, with improved charge and discharge rates.
- Biomedicine: Applications in biosensors, drug delivery, and tissue engineering.
- Coatings: Ultra-thin, strong coatings for corrosion resistance and anti-friction applications.
Single-Walled Carbon Nanotubes (SWCNT)

Learn More
 What are Carbon Nanotubes?
What are Carbon Nanotubes?
Carbon nanotubes (CNTs) have been deemed a wonder material due to their remarkable and highly unique physical and chemical properties. They have received much attention over the past decade as a promising material, particularly in the trending field of nanotechnology.
Learn more... Graphene vs Graphite
Graphene vs Graphite
Graphene is a single layer of carbon atoms arranged in a hexagonal pattern, like a sheet of paper. Graphite, on the other hand, is made up of many layers of graphene stacked on top of each other, like a stack of paper.
Read more...
References
- Carbon nanotubes for flexible batteries: recent progress and..., Zhu et al., National Science Review (2021)
- Graphene synthesis, characterization and its applications: A review., Mbayachi, V. B. et al., Results Chem. (2021)
- Carbon nanotubes: properties, synthesis, purification, and medical applications, Eatemadi, A., Nanoscale Res Lett. (2014)
- Measurement of the Elastic Properties and Intrinsic Strength..., Lee, C. et al., Science (2008)
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer