4′-Heptyl-4-biphenylcarbonitrile
CAS Number 41122-71-8
Liquid Crystals, Materials, Optoelectronic Materials4′-Heptyl-4-biphenylcarbonitrile (7CB), liquid crystal (nematic).
Nematic liquid crystal for the application of optical electronics i.e. in liquid-crystal display (LCD).
Specifications | Pricing and Options | MSDS | Literature and Reviews
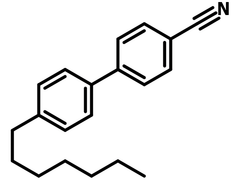
4′-Heptyl-4-biphenylcarbonitrile (7CB), CAS number 41122-71-8, has a simple structure of 1,1'-biphenyl with cyano and heptyl groups located at 4,4'-positions. 7CB belongs to the family of thermotropic liquid crystals from the cyanobiphenyl family.
Studies showed that the dispersion of CQDs in host nematic matrix can induce the quenching of photoluminescence and reduce the UV–Vis absorbance of pure nematic at a lower wavelength. Dielectric properties of the 7CB liquid crystal can change quite significantly when multiwalled carbon nanotubes are presented in the liquid crystal matrix.
Liquid crystal molecule 4′-heptyl-4-biphenylcarbonitrile can also enhance the device performance of perovskite solar cells by interacting with PbI2 to control the crystal growth orientation for fine and oriented perovskite domains for the benefit of electron transport and device stability. When compared with the pristine devices, the power conversion efficiency (PCE) of the liquid crystal based device improved from 17.14% to 20.19% with a high fill factor (FF over 80%). Also 7BC based PSCs retain 92% of their initial efficiency at 25 °C, and a relative humidity of 70% after 500 h, whereas the control devices are almost degraded completely.
Liquid crystal
With biphenylcarbonitrile core
Induce alignment
To improve perovskite solar cells performance
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
Chemical Structure
General Information
| CAS Number | 41122-71-8 |
| Chemical Formula | C20H23N |
| Molecular Weight | 277.40 |
| Full Name | 4′-Heptyl-4-biphenylcarbonitrile |
| Synonyms | 7CB, 4-Cyano-4'-heptylbiphenyl, 4'-Heptyl-4-biphenylcarbonitrile |
| Classification / Family | Biphenylcarbonitrile, Nematic Liquid Crystals |
Product Details
| Purity | >98% (1HNMR) |
| Form | White powder/crystals |
| Mesomorphic Range | 27.0 to 45.0 °C |
| Melting Point | Tm = 36.0 °C |
Pricing
| Batch | Quantity | Price |
| M2359A1 | 5 g | £130 |
| M2359A1 | 10 g | £220 |
| M2359A1 | 25 g | £440 |
MSDS Documentation
4′-Heptyl-4-biphenylcarbonitrile MSDS Sheet
Literature and Reviews
- Optical properties and zeta potential of carbon quantum dots (CQDs) dispersed nematic liquid crystal 4′- heptyl-4-biphenylcarbonitrile (7CB), F. Pandey et al., Opt. Mater., 105, 109849 (2020); DOI: 10.1016/j.optmat.2020.109849.
- Liquid Crystal Molecule as “Binding Agent” Enables Superior Stable Perovskite Solar Cells with High Fill Factor, L. Tao et al., RRL Solar, 3 (8), 1900125 (2019); DOI: 10.1002/solr.201900125.
- Liquid crystalline cyanobiphenyl homologues doped with gold nanoparticles, K. Vardanyan et al., Liq. Cryst., 39 (9), 1083–1098 (2012); DOI: 10.1080/02678292.2012.696729.
