Phenyl 1-hydroxy-2-naphthoate
CAS Number 132-54-7
Liquid Crystals, Materials, Optoelectronic MaterialsPhenyl 1-hydroxy-2-naphthoate, liquid crystal Intermediate
Intermediate for the synthesis of natural products and anti-carcinogenic compounds, liquid crystal intermediates.
Specifications | Pricing and Options | MSDS | Literature and Reviews
Phenyl 1-Hydroxy-2-naphthoate (PHN), CAS number 132-54-7, is a phenyl ester of 1-hydroxy-2-naphthoic acid. Hydroxynaphthoate derivatives are well known for their biological and medicinal properties. The 1-hydroxy-2-naphthoate moiety has been discovered in natural products such as the cytotoxic compounds 3-hydroxymollugin and 3-methoxymollugin. For their biological and medicinal properties, hydroxynaphthoates play an important role in drug discovery and they have been used as intermediates for the synthesis of the anti-carcinogenic compounds.
Research also shows that both methyl 1-hydroxy-2-naphthoate and ethyl 1,6-dihydroxy-2-naphthoate develop anti-inflammatory activity, while 1-hydroxy-2-naphthoic acid itself has been recognized as an antibacterial agent.
Monomer for drug discovery
1-hydroxy-2-naphthoate moiety has been discovered in natural products
Exhibiting solvatochromism
Attributed to the intramolecular proton transfer
Worldwide shipping
Quick and reliable shipping
High purity
>98% High purity
Chemical Structure
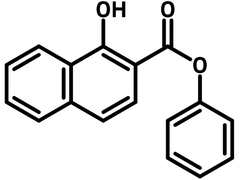
General Information
| CAS Number | 132-54-7 |
| Chemical Formula | C17H12O3 |
| Molecular Weight | 264.28 |
| Full Name | Phenyl 1-hydroxy-2-naphthoate |
| Synonyms | 1-Hydroxy-2-naphthoic acid phenyl ester, 2-Phenoxycarbonyl-1-naphthol, Phenyl 1-hydroxy-2-naphthalate |
| Classification / Family | Hydroxynaphthoate derivatives, Anti-inflammatory activity, Antibacterial |
Product Details
| Purity | >98% (1HNMR) |
| Form | Off-white powder/crystals |
| Melting Point | 93.0 to 96.0 °C |
Pricing
| Batch | Quantity | Price |
| M2367A1 | 25 g | £125 |
| M2367A1 | 50 g | £220 |
| M2367A1 | 100 g | £340 |
MSDS Documentation
Phenyl 1-hydroxy-2-naphthoate MSDS Sheet
Literature and Reviews
- Solvatochromism and intramolecular hydrogen-bonding assisted dipole moment of phenyl 1-hydroxy-2-naphthoate in the ground and excited states, I. Sıdır et al., J. Mol. Liq., 221, 972-985 (2016); DOI: 10.1016/j.molliq.2016.06.019.
- Infrared Spectrum and UV-Induced Photochemistry of Matrix-Isolated Phenyl 1-Hydroxy-2-Naphthoate, I. Sıdır et al., Photochem., 1(1), 10-25 (2021); DOI: 10.3390/photochem1010002.
- Crystal structure of phenyl 1-hydroxy-2-naphthoate, an unexpected product from an attempted aryl aryl coupling reaction, K Peters et al., Z. Kristallogr. Cryst. Mater., 210 (3), 210-211 (1995); DOI: 10.1524/zkri.1995.210.3.210.
