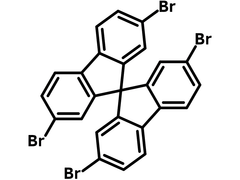
2,2′,7,7′-Tetrabromo-9,9′-spirobifluorene
CAS Number 128055-74-3
Chemistry Building Blocks, Materials, Monomers, Non-Heterocyclic Building BlocksHigh-Purity 2,2′,7,7′-Tetrabromo-9,9′-spirobifluorene
A very useful intermediate for the synthesis of semiconducting molecules, polymers with a spirobifluorene core for applications in highly efficient OLEDs and perovskite solar cells, i.e. Spiro-OMeTAD (Spiro-MeOTAD)
Specifications | MSDS | Literature and Reviews
2,2′,7,7′-Tetrabromo-9,9′-spirobifluorene (TBSBF), CAS number 128055-74-3, has a chemical structure of 9,9′-spirobifluorene core fully end-brominated benzene rings. 9,9′-spirobifluorene compounds have been known for their excellent thermal and chemical stabilities and facile solid amorphous films forming characteristics. The four bromos on each benzene ring enable further modification and extension of the core structure for more complex structures.
2,2′,7,7′-tetrabromo-9,9′-spirobifluorene can be prepared by brominating 9,9′-spirobifluorene using bromine as the oxidising agent and iron(III) chloride as the catalyst in quantitative yield. Yamamoto polymerization of 2,2′,7,7′-tetrabromo-9,9′-spirobifluorene using bis-(1,5-cyclooctadiene)nickel(0) (Ni(COD)2) as the catalyst gives thermally and chemically stable poly (SBF). Upon thermal treatment, the polymer has BET surface areas of ca. 2,000 m2 g-1.
An organic light-emitting device based on 2,2’,7,7’-tetrakis(pyren-1-yl)-9,9’-spirobifluorene (TPSBF) which was synthesised from 2,2′,7,7′-tetrabromo-9,9′-spirobifluorene as one of the starting materials, exhibited a broad-spectrum white emission showing a maximum luminescence and current efficiency of 57 680 cd m-2 and 6.51 cd A-1, with the following configuration: ITO (170 nm)/2-TNATA (15 nm)/NPB (65 nm)/TPSBF (50 nm)/Alq3 (30 nm)/LiF (0.8 nm)/Al (200 nm).
Spirobifluorene building block
For the synthesis of OLED and organic photovoltaic materials
Worldwide shipping
Quick and reliable shipping
Capped with bromides
for facile coupling reactions
High purity
>99% Purity
General Information
| CAS Number | 128055-74-3 |
| Chemical Formula | C25H12Br4 |
| Full Name | 2,2′,7,7′-Tetrabromo-9,9′-spirobifluorene |
| Molecular Weight | 631.98 g/mol |
| Synonyms | 2,2′,7,7′-Tetrabromo-9,9′-spirobi[9H-fluorene], TBSBF |
| Classification / Family | Spirobifluorene, semiconductor synthesis intermediates, low band gap polymers, OLED, OFETs, organic photovoltaics |
Chemical Structure
Product Details
| Purity | >99% (HPLC) |
| Melting Point | Tm = 395 °C - 400 °C |
| Appearance | White to off-white powder/crystals |
MSDS Documentation
 2,2′,7,7′-Tetrabromo-9,9′-spirobifluorene MSDS Sheet
2,2′,7,7′-Tetrabromo-9,9′-spirobifluorene MSDS Sheet
Literature and Reviews
- Fluorinated 9,9'-spirobifluorene derivatives as host materials for highly efficient blue organic light-emitting devices, C. Li et al., J. Mater. Chem. C, 1, 2183-2192 (2013); DOI: 10.1039/C3TC00466J.
- Molecular Glass Resists Based on 9,9′-Spirobifluorene Derivatives: Pendant Effect and Comprehensive Evaluation in Extreme Ultraviolet Lithography, J. Chen et al., ACS Appl. Polym. Mater., 1 (3), 526–534 (2019); DOI: 10.1021/acsapm.8b00235.
- A Spirobifluorene Derivative as a Single-Emitting Component for a Highly Efficient White Organic Electroluminescent Device, H. Wen et al., Chempluschem, 78(10), 1288-1295 (2013); DOI: 10.1002/cplu.201300088.
