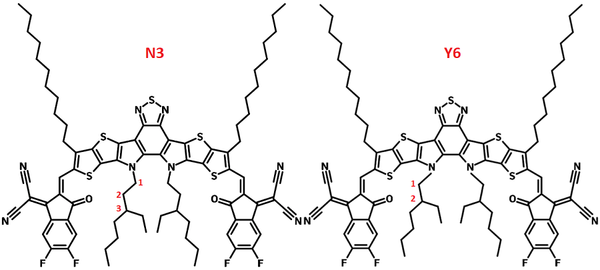
N3 - Y6N3
Materials, Non-Fullerene AcceptorsN3, small molecule non-fullerene acceptor and Y6 family member
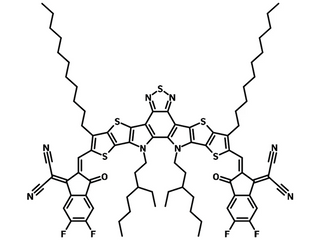
Exhibits optimum properties of domain size and crystallinity resulting in excellent device performance, Y6N3, Y6-N3, Y6-C2, 2,2'-((2Z,2'Z)-((12,13-bis(3-ethylheptyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2'',3'':4',5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile
Specifications | Pricing and Options | MSDS | Literature and Reviews
N3 (Y6-N3) is another non-fullerene acceptor that belongs to the Y6 family. The only difference between N3 and Y6 is that the location of the branching points on the inside branched side chains and a slightly longer chain (3-ethylheptyl) for N3, while Y6 has classic 2-ethylhexyl branched side chains. With 3rd-position branched alkyl chains, N3, exhibits optimum properties in terms of domain size, crystallinity, and more dominant face-on orientation of the π-π stacking.
N3 has a clear solid-state aggregation transition at 82 °C. The shifting of the branching position of the alkyl chains increases the solubility of the molecule significantly from 40 mg/mL for Y6 to 64 mg/mL for N3 in chloroform.
Optimised photovoltaic devices using a binary acceptor system with N3:PC71BM as the acceptors and PBDB-T-2F (PM6) as the polymer donor shows efficient device performance with power conversion efficiencies (PCEs) of up to 16.74% (device 1), and 18.69% while D18-Cl:N3:PC61BM (1:1.4:0.1) is used as the active layer materials (device 2).
Device structure 1: ITO/PEDOT:PSS/PM6:N3:PC71BM/PNDIT-F3N/Ag.
| Thickness (nm) | VOC (V) | JSC (mA cm-2) | FF (%) | PCE (%) |
| 110 | 0.850 | 25.71 | 76.6 | 16.74 |
Device structure 2: ITO/PEDOT:PSS/D18-Cl:N3:PC61BM (1:1.4:0.1)/PDIN/Ag.
| Thickness (nm) | VOC (V) | JSC (mA cm-2) | FF (%) | PCE (%) |
| 114 | 0.849 | 28.22 | 78.0 | 18.69 |
Y6 (BTP-4F) from Ossila was used in the high-impact paper (IF 29.37), Triplet-Charge Annihilation in a Small Molecule Donor: Acceptor Blend as a Major Loss Mechanism in Organic Photovoltaics, J. Marin-Beloqui et al., Adv. Energy Mater., 2100539 (2021); DOI: 10.1002/aenm.202100539.
General Information
| CAS Number | N/A |
| Chemical Formula | C84H90F4N8O2S5 |
| Purity | >99% (1H NMR) |
| Full Name | 2,2'-((2Z,2'Z)-((12,13-bis(3-ethylheptyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2'',3'':4',5']thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile |
| Molecular Weight | 1479.98 g/mol |
| HOMO / LUMO | HOMO = -5.65 eV, LUMO = -4.10 eV [1] |
| Synonyms | Y6N3, Y6-N3, Y6-C2 |
| Classification / Family | NFAs, n-type non-fullerene electron acceptors, Organic semiconducting materials, Low band-gap small molecule, Small molecular acceptor, Organic photovoltaics, Polymer solar cells, NF-PSCs |
Chemical Structure
Pricing
| Batch | Quantity | Price |
| M2324A1 | 50 mg | £240 |
| M2324A1 | 100 mg | £380 |
| M2324A1 | 250 mg | £780 |
*4 - 5 weeks lead time
MSDS Documents
Literature and Reviews
- Alkyl Chain Tuning of Small Molecule Acceptors for Efficient Organic Solar Cells, K. Jiang et al., Joule, 3 (12); 3020-3033 (2019); DOI: 10.1016/j.joule.2019.09.010.
- 18.69% PCE from organic solar cells, K. Jin et al., J. Semicond., 42(6), 060502 (2021); DOI: 10.1088/1674-4926/42/6/060502.
- Low Temperature Aggregation Transitions in N3 and Y6 Acceptors Enable Double-Annealing Method That Yields Hierarchical Morphology and Superior Efficiency in Nonfullerene Organic Solar Cells, Y. Qin et al., Adv. Funct. Mater., 2005011 (2020); DOI: 10.1002/adfm.202005011.
Related Products
Semiconducting polymers for bulk heterojunction, OPV, OLED, OFET and perovskite interfaces and solar cell research.

 N3 MSDS Sheet
N3 MSDS Sheet
