HTL Material to Improve Charge Extraction in OLED/OFET Devices
High quality polymer available with free shipping on qualifying orders
Specifications | MSDS | Literature and Reviews
Methyl or fluoro substituted poly(triarylamines) (PTAAs) are electron rich with diphenylamine as the the backbone and substituted phenyl as side chains. Substituted PTAAs are originally used as hole transport layer (HTL) materials in OLED/OFET devices to improve charge extractions. PTAA can also act as electron blocking layer to reduce the chances of electron–hole recombination and energy loss in electronic devices. Fluorinated PTAA, 1F-PTAA and 2F-PTAA, can effectively downshift the HOMO levels of PTAA derivatives due to the electron deficiency of fluorine, resulting in an increase of VOC hence the power conversion efficiency (PCE) of the device.
Enhanced PCE
For high efficiency devices
Reduced Energy Loss
Reduces electron–hole recombination
High Quality
High quality and electron rich
Hole Transport Layer
Improved charge extraction
PTAA from Ossila was used in the high-impact paper (IF 30.85), Multiply Charged Conjugated Polyelectrolytes as a Multifunctional Interlayer for Efficient and Scalable Perovskite Solar Cells, E. Jung et al., Adv. Mater., 2002333 (2020); DOI: 10.1002/adma.202002333.
A range of substituted poly(triarylamine)'s (PTAA's) for use with small molecules for solution-processed high-mobility films. While PTAA has a relatively modest mobility on its own, it has been gaining interest when used in conjunction with small molecules to provide the combination of good coating parameters and high mobility [1,2].
Note: We also have a PTAA family member (PTAA for perovskites) for perovskite solar cell applications.
Specifications
| Name | Batch number | Mw | Mn | PDI |
|---|---|---|---|---|
| 4-Fluoro PTAA | PE10-338-12 | 6,000 g/mol | 4,020 g/mol | 1.5 |
| Dimethyl PTAA | PE3-298-27B | 6,312 g/mol | 4,390 g/mol | 1.4 |
| 2,4 Difluoro PTAA | PE-338-8 | 4,712 g/mol | 3,504 g/mol | 1.34 |
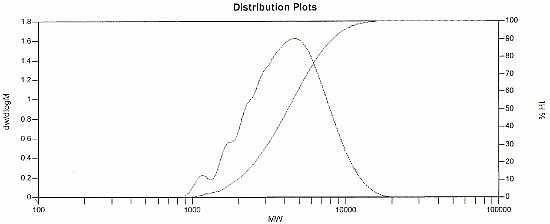
2,4 Difluoro PTAA Distribution Plot
MSDS Documentation
Literature and References
- Solvent and polymer matrix effects on TIPS-pentacene/polymer blend organic field-effect transistors Do Kyung Hwang et al., Journal of Materials Chemistry, V22, P5531-5537 (2012).
- Solution-Processed Small Molecule-Polymer Blend Organic Thin-Film Transistors with Hole Mobility Greater than 5 cm 2 /Vs J. Smith et al., Advanced Materials, V24, P2441-2446 (2012).
