What is PEDOT:PSS?
PEDOT:PSS is a blend of two distinct polymers: poly(3,4-ethylenedioxythiophene) (PEDOT) and polystyrene sulfonate (PSS). This combination forms a p-type semiconductor which is highly valued for its ability to conduct electricity and its ease of processibility.
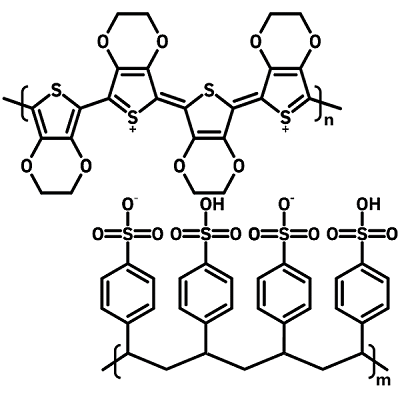
PEDOT is highly conductive with impressive mechanical flexibility, while PSS offers stability and allows dispersion in water. Together, they create a material that is transparent in the visible spectrum and exceptionally conductive. This makes PEDOT:PSS an extremely attractive material for use in many optical and electronic devices.
What is PEDOT?
PEDOT is a conjugated polymer which is formed of 3,4-ethylenedioxythiophene (EDOT) monomers. These monomers are joined at 2,5-positions of each five-membered thiophene ring to create linear polymer chains.
Poly(3,4-ethylenedioxythiophene) (PEDOT) is the conductive component of PEDOT:PSS. However, pristine PEDOT is insoluble in many solvents. It therefore requires the presence of a stabilizer and dopant molecule such as PSS in order to be synthesized in water or other solvents.
What Is PSS?
Polystyrene sulfonate (PSS) is a polymer surfactant with a styrene backbone and a sulfonate group (-SO3H) acting as the pending unit.
PSS is not conductive. However, PSS helps disperse and stabilize PEDOT in water and other solvents so it can be solution processed. PSS will coat insoluble PEDOT molecules, forming nano-size particles of PEDOT:PSS, which form a stable colloidal dispersion in water. PSS will also improve the stability of PEDOT:PSS.
PSS is currently the most successful PEDOT counterpart dopant.
Why Is PEDOT:PSS Important?
PEDOT:PSS is one of the most popular conductive polymers due to its impressive blend of material properties. PEDOT: PSS’s many attractive qualities include:
- High mechanical flexibility
- Can be easily processed and deposited into a thin film
- High optical transparency in the visible light region
- Great thin film stability under ambient conditions
- Low surface roughness
- High work function
- High range of conductivities (10-4-103 S cm-1)
- Low cost compared to other conjugated polymers
PEDOT Applications
PEDOT:PSS is by far one of the most successful conductive polymers. It is compatible with many solution processing techniques including dip coating, spin coating, slot die coating, spray coating, doctor-blade coating, inkjet printing, screen printing, etc. This means you can coat a range of substrates with PEDOT:PSS solutions, rigid or flexible, regular or irregular.
In scientific research, PEDOT:PSS uses span from fundamental research to practical applications, including organic photovoltaics (OPVs), organic light-emitting diodes (OLEDs), and sensors, biosensors, supercapacitors, antistatic coatings, electrically conducting coatings, thermoelectric materials, field effect transistors, and active materials for electrochromic devices.
Perovskite Photovoltaics
PEDOT:PSS has been used as a hole extraction material in inverted PSC devices. This material facilitates the extraction of charge carriers at the interface between the transparent conductive oxide and the active perovskite layer.
Organic Photovoltaics
PEDOT:PSS has long been used in OPV fabrication, often used in combination with materials such as P3HT and PCDTBT. To this day, it remains a popular material choice for polymer solar cell research.
Organic Light Emitting Diodes
The use of PEDOT:PSS in organic light emitting diodes, as a well-established standard hole injection material, has been widespread for over a decade. Recent work still uses PEDOT:PSS due to its deep work function. This allows for efficient charge injection into white emitting polymers as well as host materials for thermally activated delayed fluorescence materials.
Transparent Conductors
PEDOT:PSS is a potential replacement for expensive transparent metal oxides, such as ITO and FTO. Its effectiveness in both organic photovoltaic and perovskite photovoltaic devices has been demonstrated. In addition, in combination with metallic grid structures, it is possible to achieve sheet resistances comparable to metallic films.
It is also widely used for industrial applications. For example, both aqueous and non-aqueous deep-blue PEDOT:PSS dispersions are now commercially available from Heraeus Clevios™ and Agfa Orgacon™.
Types of PEDOT:PSS
One of the most important differences between different PEDOT solutions is the ratio of PEDOT to PSS. Changing the ratio of PEDOT to PSS can drastically affect the properties of your PEDOT material.
| Ratio of PSS-to-PEDOT | Example | Conductivity | Stability of Suspension | Potential Application |
|---|---|---|---|---|
| High | CH 8000 | Low | High | Use in OLEDs to reduce unwanted charge drift. |
| Low | PH 1000 | High | Low | Electrode or transport layer in device |
Therefore, it is important to choose the right ratio of PSS-to-PEDOT for your desired application.
Solvent choice is also important when selecting a PEDOT complex. Most PEDOT:PSS solutions are dispersed in water. However, other PEDOT complexes use alternative stabilizers to PSS, enabling dissolution in different solvents. This may be a useful alternative if you are working with materials that are hydrophobic or that degrade when exposed to water.
Some PEDOT:PSS solutions also incorporate additives to adapt the solutions for specific purposes, such as for deposition onto fabrics or to change its thermoelectric properties
PEDOT:PSS Structure

Although the characteristics of PSS are well defined, PEDOT:PSS has a much more complex structure. The intimate association of two polymers in solution leads to some interesting dynamics in the suspension and in the subsequent thin film. One term for this intricate combination of PEDOT and PSS is a “chain structure entanglement”.
During synthesis, it seems that PEDOT synthesizes onto the PSS template. In the resulting suspension, small segments of PEDOT are in close contact with PSS bundles bound by Coulomb forces. These entangled PEDOT:PSS bundles form a colloidal suspension of gel particles in water where:
- Hydrophobic PEDOT populates the inner core
- Hydrophilic PSS makes up the outer layer
When deposited into a thin film, the film morphology is driven by this chain structure entanglement. The PEDOT:PSS gel particles will form pancake-like structures, with these bundles stacking on top of one another in the thin film.
The morphological and electronic properties of a PEDOT:PSS film depend on the formation of these gel particles and their stacking. Therefore, film properties are dependent on many factors including:
- PEDOT and PSS ratio and synthesis method
- PEDOT:PSS deposition and processing methods
- Any additives in the precursor solution
- Any pre- and post-treatment of the deposited films
References
Huseynova, G., Hyun Kim, Y., Lee, J.-H., & Lee, J. (2019). Rising advancements in the application of PEDOT:PSS as a prosperous transparent and flexible electrode material for solution-processed organic electronics. Journal of Information Display, 21(2), 71–91. https://doi.org/10.1080/15980316.2019.1707311
Wang, Z., & Liu, R. (2023). PEDOT:PSS-based electrochromic materials for flexible and stretchable devices. Materials Today Electronics, 4, 100036. https://doi.org/10.1016/j.mtelec.2023.100036
Read More
Contributors
Written by
Application Scientist
Diagrams by
Graphic Designer

