What is a Redox Reaction?

A redox reaction, also referred to as an oxidation-reduction reaction, involves the loss or gain of electrons. The electrons are transferred between two species and their oxidation number changes as a result. Species that can undergo redox reactions include atoms, ions and molecules. Redox reactions are common throughout nature, but in scientific research, they are particularly important because they play a critical role in processes such as energy transfer, metabolic pathways, and cellular respiration. Redox reactions are fundamental in fields like electrochemistry, where they drive processes in batteries and fuel cells.
Synthetic redox reactions typically happen in electrochemical cells with working, reference and counter electrodes. Anodic and cathodic working electrodes are the site of electron transfer.
A + B ⇌ A+ + B-
- Oxidation happens on the working electrode if it is driven to a positive potential compared to the reference electrode. When the electrons in the electrode are at a higher energy than the lowest unoccupied molecular orbital (LUMO) of the redox active species in the system the electron is transferred to the redox species.
A ---> A+ + e-
- Reduction reaction will happen when a negative potential is applied to the working electrode.
B + e- ---> B-
Faradaic current: electrical current generated by the transfer of charges as a result of redox reactions.
What happens during a redox reaction?
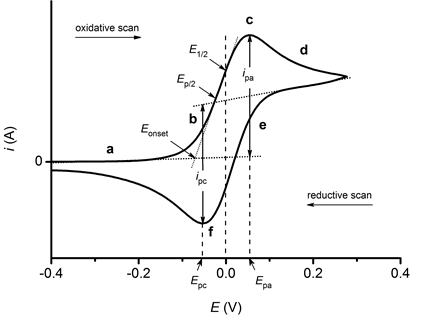
In an electrochemical cell, when the potential (voltage) reaches the point where an oxidation or reduction is induced, current starts to flow. The point at which the current starts is the onset oxidation or reduction potential (Eonset). This is shown below where Epa is the anodic peak potential, Epc is the cathodic peak potential and E1/2 is the half-wave potential:
E1/2 = (Epa + Epc) / 2
Oxidation: When the applied potential rises past the half-wave potential, oxidation becomes thermodynamically favorable. The current also continues to increase. The oxidation process, however, becomes limited by rate of diffusion process to the electrode surface. The redox active species within the electrochemical cell struggles to reach the electrode fast enough. This is characterized by a drop of the current (resembling a duck beak) as fewer species are oxidized per second.
For a chemically reversible charge transfer process, the electrode potential sweep is then reversed and scanned back to the initial value. Up to this point oxidation can still take place.
Reduction: When the potential becomes sufficiently negative the reversal results in the appearance of the cathodic peak. It exceeds the half-wave potential due to the reduction of the electrochemically generated species generated during the first sweep. The reversed potential sweep is mostly associated with a reductive (negative) current.
As more oxidized species become reduced the current increases, characterized by the duck tail. As this happens a cyclic voltammogram becomes complete.
What does Reduction Potential mean?
The reduction potential is a measure of the tendency of a chemical species to gain electrons and be reduced. It is also known as the redox potential or electrode potential. Reduction potential is usually measured in volts (V) relative to a standard reference which is assigned a potential of 0.00 V.
Reduction potential tells us how likely a species is to be reduced when paired with another substance in an electrochemical cell. The more positive the reduction potential, the greater the species' affinity for electrons and therefore it is more likely to be reduced. Conversely, a negative reduction potential indicates the species is less likely to undergo reduction. Instead, there is a higher likelihood that the species acts as an electron donor (or undergoes oxidation).
- Positive reduction potentials are associated with oxidizing agents that readily gain electrons.
- Negative reduction potentials are associated with reducing agents that readily lose electrons.
Reduction potentials are critical in determining the direction of electron flow in reactions and play a key role in fields such as electrochemistry, biochemistry, and environmental science.
Why is reduction potential important in batteries?
Reduction potential dictates how easily species gain or lose electrons. During the discharge of batteries, the flow of electrons from the anode to the cathode through an external circuit is essential for generating electrical power. The faster this happens the better. A large difference in reduction potentials between the anode and cathode materials results in a high overall cell voltage. This high voltage translates to greater energy output per unit charge, which is ideal for powering devices more effectively.
Lithium has a very low reduction potential of -3.04 (Li+ + e ⇌ Li). This means it is an excellent electron donor, a property highly desired in batteries. Lithium's low reduction potential maximizes voltage and energy density, enhances efficiency, and supports rechargeability.
Lithium-based materials make good cathode active materials because they can store lithium ions and release them when needed. During the battery's discharge, these lithium ions flow from the anode to the cathode through the electrolyte, while electrons flow through an external circuit to power devices. The lithium-based cathode material can efficiently accept these electrons and "capture" the lithium ions, completing the circuit and providing stable energy output.
Electrochemical Cells

Learn More
 Cyclic Voltammetry: Theory and Setup
Cyclic Voltammetry: Theory and Setup
Cyclic voltammetry is an electrochemical technique for measuring the current response of a redox active solution to a linearly cycled potential sweep between two or more set values.
Read more... Troubleshooting Cyclic Voltammetry
Troubleshooting Cyclic Voltammetry
With our modern potentiostat and software package, the method is straight-forward to perform. But if run into unexpected problems, this guide can help you to identify the source.
Read more...Further Reading
- Sandford, C. et al. (2019). A synthetic chemist's guide to electroanalytical tools for studying reaction mechanisms, Chem. Sci, 10. doi:10.1039/C9SC01545K
- Elgrishi, N. et al. (2018). A Practical Beginner’s Guide to Cyclic Voltammetry, J. Chem. Educ., 95. doi:10.1021/acs.jchemed.7b00361