UV Ozone Cleaning: Theory and Application

UV ozone cleaning relies upon the use of a high-intensity UV light source, which illuminates the surface to be cleaned with two specific wavelengths of light. Low pressure mercury vapor discharge lamps are typically used, like the synthetic quartz UV grid lamp in the Ossila UV Ozone Cleaner, which have two dominant emission peaks at 184 nm and 254 nm. Upon irradiation, molecular oxygen present in the air is dissociated by radiation below 200 nm in length. This results in the formation of two radicals of oxygen. These radicals go on to react with further molecular oxygen forming molecules of ozone.
At the same time, light at 254 nm is used to excite organic species present on the surface of the sample. This process increases the reactivity of the contaminants with ozone. Upon reacting, the material is cleaned from the surface.
UV Ozone Cleaner

How Does UV Ozone Clean Samples?
UV ozone cleaning is a photo-sensitized oxidation process in which organic molecules in their excited state chemically react with ozone molecules, resulting in the cleaving of bonds and the dissociation of molecules from the surface. The process use a high intensity UV light source which has two dominant emission peaks at 185 nm and 254 nm. These two wavelengths are responsible for different processes, which ultimately result in the cleaning of the surface.
Radiation below 200 nm is strongly absorbed by molecular oxygen. The energy of the absorbed photon is enough to break the oxygen-oxygen double bond, resulting in the formation of two free radicals of oxygen (O•). These free radicals can subsequently react with molecular oxygen producing ozone molecules (O3).
UV radiation at 254 nm is readily absorbed by organic species that are present on the surface of many substrates. The exciton that is formed will be in a highly energetic state, and the energy may also be high enough for certain molecules to make organic radicals.
The excited states and organic radical species present on the surface readily react with ozone present within the atmosphere, resulting in the formation of volatile species such as carbon dioxide, water, molecular nitrogen, and short chain organic compounds. These volatile compounds can easily desorb from the surface under atmospheric conditions, leaving a pristine surface.
How Does UV Ozone Alter Surface Energy?
UV ozone treatment alters the surface energy of samples via two methods. The first of these is through the removal of low energy contaminants from the surface. These are typically organic atmospheric contaminants that have adsorbed onto the surface of a substrate. The second way is through treatment of the surface and the formation of high energy bonds on the surface of the samples.
The removal of contaminants is done via the photo-oxidation process. This process results in the desorption of contaminants from the surface due to the chemical break down of the organic material. The underlying substrate is typically a higher energy surface, such as a ceramic or a metal, which results in the surface energy of the sample increasing in comparison to when it was untreated. This treatment does not last forever as over time organic contaminants will begin to reabsorb back onto the surface, slowly decreasing the surface energy.
The second way that UV ozone treatment works to improve the surface energy is via the formation of hydroxyl functional groups on the surface of the substrate. During the irradiation process, light at 253.7 nm can break down water molecules, resulting in the formation of OH and O free radicals. Hydroxyl free radicals will typically react with ozone present to form water and oxygen, however, when the UV degradation of water occurs near the surface of the sample, the hydroxyl free radical can react with the surface, forming a functional group. This functional group has a high bonding energy, resulting in an increase in the surface energy of most surfaces.
UV Ozone Applications
UV ozone cleaning is a versatile technique which can be applied to a large array of materials to provide surface cleaning and treatment. It can also be used for a variety of other applications which require either the presence of ozone or UV light, meaning that the method sees a wide range of uses across multiple disciplines. The two main applications for the technique are for surface cleaning and surface treatment.
Surface Cleaning
Surface cleaning using UV ozone cleaning is typically done as the final step in a cleaning procedure to remove residual organics that are present on the surface of a sample. The process results in an atomically clean surface, free from any organic contaminants. Contaminants that can be cleaned with UV ozone cleaning include photoresists, human skin oils, plastic surface / silicon oil residues, resins, cleaning solvent residues, and solder flux.
Surface Treatment
During the cleaning process, the formation of ozone and oxygen radicals can result in a reaction with water molecules present in the air. This results in the formation of hydroxide radicals. These short lived, highly reactive species can react with bonds on the surface of substrates, resulting in the formation of high energy hydroxide groups. This can help with preparation of samples by increasing the surface energy of a substrate.
Other applications of UV ozone cleaning include UV curing, UV chemical reactions, the removal of surface monolayers, oxidation of surfaces, and micropatterning. Common materials that can be treated using UV ozone cleaning include:
Removal of Surface Monolayers
In this application, we used the UV ozone cleaner to remove a surface layer of n-octadecyl trichlorosilane (OTS) to improve the wetting of water-based solutions on a silicon substrate.


OTS is an organic molecule that is used in the fabrication of organic field effect transistors to improve the electrical properties of deposited films. It consists of a trichlorosilane group, which reacts with the native oxide of silicon to form three siloxane bonds with the surface. These bonds repeat across the surface of the silicon substrate until the entire surface is covered in a monolayer of OTS.
The long hydrocarbon chains result in the substrate having a very low surface energy. Wetting of high surface energy solvents, such as water, therefore becomes impossible, and the contact angle of deposited droplets is high. The OTS-treated substrate was exposed to UV ozone for approximately 10 minutes to clean the surface of the octadecane carbon chains. The treatment increased the surface energy enough to allow complete wetting of the water droplet on the substrates surface.
Treating Plastic Surfaces
Surface treatment can be used to improve the surface energy of a substrate. The treatment time (length of exposure to ozone) can vary the degree of change in surface energy.
For example, plastic substrates have very low surface energy due to the abundance of C-H bonds and other similar low energy bonds. As a result, due to the poor wetting that occurs, coating thin films from solutions that have high surface tension solvents can be difficult.
One method to assess the degree of wetting is to look at the contact angle that a droplet makes on the surface of the substrate. The lower the contact angle (measurable with an Ossila Contact Angle Goniometer) that a particular solvent makes, the better the wetting is. UV ozone cleaning can be used to treat the surface to improve the wetting of solvents.
During the process of UV ozone treatment, ozone reacts with surface bonds, breaking down the organic groups and eventually releasing volatile species. During the process, intermediate steps occur in which low-energy bonds such as the C-H bond are replaced with higher energy groups such as C-OH.
UV Ozone Cleaner

Learn More
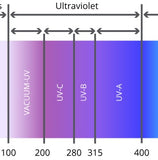 What is UV Sterilization?
What is UV Sterilization?
Ultraviolet (UV) sterilization is a disinfecting technique that uses UV light to kill or damage microorganisms, such as bacteria, viruses, and fungi.
Read more...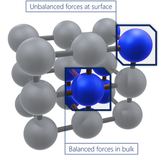 Surface Energy: Formula & Definition
Surface Energy: Formula & Definition
Surface free energy is a measure of the excess energy present at the surface of a material, in comparison to at its bulk. It can be used to describe wetting and adhesion between materials, but is not often used quantitatively.
Read more...